
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
The following compound is a minor component of scorpion venom. Draw the most stable form of the Newman projection along the C4-C5 bond.
Identify the scorpion species
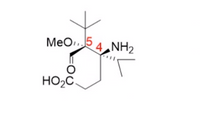
Transcribed Image Text:MeO5 NH2
4
HO2C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- i) How many lone pairs of electrons are present? ii) What is the configuration of the chiral centre? iii) What is the hybridization of the nitrogen atom in the nitrile moiety? iv) How many pi bonds are there?arrow_forwardWhich of the following is a wedge and dash structure for the following Newman projection? ed swer IV 111 CH37 H Et H CH3 Et ||| IVarrow_forwardGiven the following structure and numbering: H CO,H 3 H3C 1 A (a) Label the stereocenters of A as R or S and draw a Fischer Projection of the enantiomer of A with C1 on top and C4 on bottom. (b) Draw a Newman Projection of a diastereomer of 4 looking down the C2-C3 bond with C2 in front.arrow_forward
- Convert the following chair cyclohexane structure into a valid flat line-bond drawing: Brcl H3CO Br" Br" CI "OCH3 CI Le Dian D "OCH3 Br OCH 3 Bri "OCH3 CI CIarrow_forward1. Draw a Newman projection looking down the indicated bond for the following molecules a) H₂N 2 3 about the C2-C3 bond b) 3 Br 4 about the C3-C4 bondarrow_forwardWill the following compound show any optical activity if there is restricted rotation along the central C-C bond? What will happen to the optical activity at elevated temperatures as the rotation becomes less restricted? CH3 CI H Cl CH3arrow_forward
- Draw the substituents in the specified locations on the given cyclohexane ring to show the most stable chair conformation. Be sure that the bonds are drawn to clearly show whether they are axial or equatorial. Substituent CH3 Position 2 3 4arrow_forwardixarrow_forwardWhich labeled carbons (C1-C4) is/are sp3-hybridized? A)carbon two and four B)carbon three C)carbon one D)carbon two E)carbon one and threearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY