
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
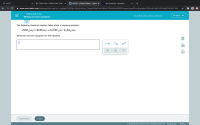
Transcribed Image Text:The following chemical reaction takes place in aqueous solution:
ZNSO,(aq)+2 KOH(aq) →Zn(OH),(s)+K,SO4(aq)
Write the net ionic equation for this reaction.
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write the balanced half-reactions for the following redox reactionsarrow_forwardIn the reaction FeCl 3 (s) + H2 (g) → 3HCI (g) + Fe (s), Cl acts as a reducing agent neither a reducing nor oxidizing agentarrow_forwardBalance the following equation in basic solution: 3CI (aq) +2H* + 2NO; (aq) → 3CIO (aq)+2NO(g) + H2Oarrow_forward
- Balance the following oxidation-reduction reaction that occurs in an acidic solution using the half-reaction method. I(aq) + Clo-(ag) → I3¯(aq) + CI-(aq) HOW DO WE GET THERE? For the reduction half-reaction, which half-reaction correctly balances the elements on both sides? ClO (aq) + H2O(1) → CI-(aq) + 2 H*(aq) clo-(aq) → Cl-(aq) 2 H+(aq) + ClO-(aq) → Cl-(aq) + H20(I) Clo-(aq) → CI-(aq) + H20(1) Clo-(aq) → Cl(aq) + H20(1) + 2 H*(aq) Check Next (4 of 6)arrow_forwardThe following reaction occurs in acidic solution. As, O6(s) + MnO, (ag)AsO (aq) + Mn²* (aq) What is the sum of the coefficients in the balanced equation?arrow_forwardUse the relative strengths of nonmetals and metals as oxidizing and reducing agents, as indicated in the following unbalanced equations, to construct a table of half‐reactions. Ag (s) + Br2 (l) → AgBr (s) Ag (s) + I2 (s) → no evidence of reaction Cu2+ (aq) + I‐ (aq) → no redox reaction Br2 (l) + Cl‐ (aq) → no evidence of reactionarrow_forward
- 6. The following reaction forms a white precipitate. Is this a redox reaction? AgNO3(aq) + HCl(aq) → HNO3(aq) + AgCl(s)arrow_forwardSelect the oxidizing and reducing agent for the reaction represented by the following line notation: Pt(s)|H2(g)|H+(aq)||Cu2+(aq)|Cu(s)arrow_forwardWhat type of reaction is the following reaction: M(s) + 2 LX(aq) → 2 L(s) + M(aq) O Combustion ● Redox Precipitation Surprised Acid-Basearrow_forward
- Consider the following net ionic equation: MnO,(aq) + s²- (aq) –→ MnO,(s) + S(s)arrow_forwardIs the reaction below an example of a REDOX reaction? 6 HF(aq} + Al(OH)3[5) + 3 NAOH(aq} → NagAIF¿(s) + 6 H2O[I}arrow_forwardThe following chemical reaction takes place in aqueous solution: AGNO3(aq)+KBr(aq) →AGB1(s)+KNO3(aq) Write the net ionic equation for this reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY