
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
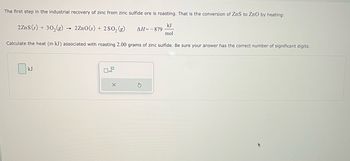
Transcribed Image Text:The first step in the industrial recovery of zinc from zinc sulfide ore is roasting. That is the conversion of ZnS to ZnO by heating:
2 ZnS (s) + 30₂(g) → 2ZnO(s) + 2SO₂ (g)
kJ
mol
Calculate the heat (in kJ) associated with roasting 2.00 grams of zinc sulfide. Be sure your answer has the correct number of significant digits.
kJ
0x10
X
ΔΗ= - 879
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 2.30 kg of water at 35.8 °C. During the reaction 74.9 kJ of heat flows out of the bath and into the flask. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions 1 is 4.18 J.g •K`1 ▪ Be sure your answer has the correct number of significant digits.arrow_forwardA 0.2415 g sample of solid magnesium is burned in a constant-volume bomb calorimeter that has a heat capacity of 3024 The temperature increases by 1.978 °C. Part 1 of 2 kJ Calculate the heat associated with the burning of Mg in Be sure your answer has the correct number of significant digits. g Part 2 of 2 kJ g ☐ x10 ☑ Calculate the heat associated with the burning of Mg in kJ mol Be sure your answer has the correct number of significant digits. KJ mol x10 ☑arrow_forwardWhen 6.54 grams of Zn is placed in 500.0 mL of 1.00 M CuSO4(aq) in a coffee cup calorimeter, it reacts completely to displace copper. The temperature of the solution rises from 20.0˚C to 30.4˚C. Assume the coffee cup itself gains no heat and that the solution has the same density (1.00 g/mL) and specific heat (4.184 J/g˚C) as pure water. (a) How much heat does the solution gain during this reaction? (in J)arrow_forward
- A chemist carefully measures the amount of heat needed to raise the temperature of a 1.10kg sample of a pure substance from −6.3°C to 10.7°C . The experiment shows that 8.40kJ of heat are needed. What can the chemist report for the specific heat capacity of the substance? Be sure your answer has the correct number of significant digits.arrow_forwardExpress the quantity in the unit indicated and designate whether this heat is gained or lost by the substance a. The quantity of heat in calories when 162.5 grams of water undergoes a temperature decrease from 45.84 C to 40.00 C. b. The quantity of heat in Kilojoules when 43.5 Kg of chloroform ,CHCl3 has its temperature changed from 16.8 C to 22.3 C ( sp ht of CHCl3 = 0.971 KJ/kg- C)arrow_forwardA series of equal-mass samples of the five materials listed in the table are brought from room temperature to 100. °C. Which sample required the least amount of energy (J) to reach the final temperature? Material Specific heat (J/g•°C) Al (aluminum) 0.903 Pyrex glass 0.75 Fe (iron) 0.449 Pb (lead) 0.128 water; H2O (l) 4.18arrow_forward
- A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 9.50kg of water at 38.9°C. During the reaction 83.3kJ of heat flows out of the bath and into the flask. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18J·g−1·K−1. Be sure your answer has the correct number of significant digits.arrow_forwardIn the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used to determine the specific heat of a solid, or to measure the energy of a solution phase reaction.A student heats 62.45 grams of platinum to 99.18 °C and then drops it into a cup containing 84.71 grams of water at 23.23 °C. She measures the final temperature to be 25.02 °C.The heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.66 J/°C.Assuming that no heat is lost to the surroundings calculate the specific heat of platinum.arrow_forwardHow much heat is given off to the surroundings when 6 g of aluminum reacts according to the following equation? Express your answer correctly rounded to two decimal places.2 Al + Fe2O3 Al2O3 + 2 Fe ΔH°rxn = -849 kJ. kJIf all of the heat generated by the reaction was absorbed by one liter of room temperature water, what would be the highest temperature the water could reach? Express your answer correctly rounded to two decimal places. °CWhile writing this question, I had to take into account that the final temperature reachable in the previous question could not exceed the boiling point of water. Given this information, what is the highest whole number amount of aluminum that could have been used for this question? Enter only a number for your answer. garrow_forward
- A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 9.80kg of water at 34.0°C . During the reaction 139.kJ of heat flows out of the bath and into the flask. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18·J·g−1K−1 . Be sure your answer has the correct number of significant digits. °Carrow_forwardOh no! Billy forgot that his calorimeter should have 2 coffee cups to contain all the heat. If Billy dropped a 25.0 g piece of solid iron (Cs = 0.449 J/g°C) at 398 K in a coffee cup containing 25.0 mL of water at 298K and because of his mistake, 21% of the heat that should be transferred from the iron to the water is lost to the atmosphere, calculate the final temperature of the iron water mixture?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY