
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
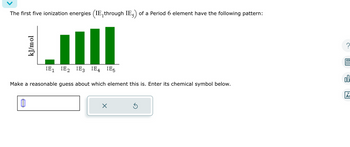
Transcribed Image Text:The first five ionization energies (IE, through IE) of a Period 6 element have the following pattern:
kJ/mol
IE₁ IE₂ IE3 IE4 IE5
Make a reasonable guess about which element this is. Enter its chemical symbol below.
X
Ś
?
00
18
Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- 11. How do the core charges for H, Li, and Na compare to each other? Based on this answer and their respective ionization energies, which species has the valence shell with the largest radius? Which has the valence shell with the smallest radius? 12. Can you deduce a trend in ionization energy as you move from left to right across a period (row)? Can you deduce a trend in core charge as you move from left to right across a period (row)? Explain.arrow_forwardHow many electrons are in the most likely ion formed from an atom located in group 5A and period 3? O 12 O 16 18arrow_forwardWhich of the following elements has the largest second ionizationenergy (IE2)? B O F Na Liarrow_forward
- Which of the following atoms and ions is (are) isoelectronic with Si: Ar, S2+, Ne, Al3+, P3−, As3+?arrow_forwardConsider the following set of successive ionization energies: IE1=578kJ/mol IE2=1,820kJ/mol IE3=2,750kJ/mol IE4=11,600kJ/mol To which third-period element do these ionization values belong?arrow_forwardWhich of the following atoms is the largest? O Rb O Li OK O Cs O Naarrow_forward
- Indicate which one statement is not true by checking the box in front of the untrue statement. The ionic radius of a sodium ion is larger than the atomic radius of a sodium atom. The ionic radius of a chloride ion is larger than the atomic radius of a chlorine atom. The ionic radius of a calcium ion is smaller than the atomic radius of a bromide ion. The ionic radius of a calcium ion is smaller than the atomic radius of a potassium ion. The ionic radius of a nickel(II) ion is smaller than the atomic radius of a nickel atom.arrow_forwardAn atomic cation with a charge of +1 has the following electron configuration: 1s²2s²2p5 What is the chemical symbol for the ion? How many electrons does the ion have? How many 2p electrons are in the ion? 1 1 Xarrow_forwardWhich of the following elements has the highest first ionization energy? Ве Na Li Rb Karrow_forward
- Which of the following elements would you expect to have chemical properties most similar to those of sulfur (S)? Na Sr Se CIarrow_forwardWhich of the following atom has the lowest ionization energy (IE1)? A Li B D C N Farrow_forwardSelect the element that best fits. You may use elements more than once. All elements are not used.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY