
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
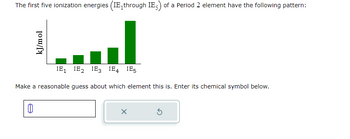
Transcribed Image Text:The first five ionization energies (IE, through IE) of a Period 2 element have the following pattern:
kJ/mol
IE₁ IE₂ IE3 IE4 IE5
Make a reasonable guess about which element this is. Enter its chemical symbol below.
Ś
Expert Solution
arrow_forward
Step 1
Given:
Pattern of first five ionization energies (IE1 to IE5) of a Period 2 element
We have to find the element and write its chemical symbol
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- description An element in Period 3 and Group 4A. A semimetal in Group 8A. An element in the nitrogen family with a higher atomic number than argon. A main-group element in Period 3. Does any element with Z ≤ 92 match the description? Yes Yes Yes Yes No No No NO If you checked yes, give the symbol of an element with Z ≤ 92 that matches. 0 0 0arrow_forwardDescribe the process happening in this image in as much detail as possible and use the periodic table to identify the elements.arrow_forwardTwo main-group elements are highlighted in the outline of the Periodic Table below: What can you say about these elements without knowing exactly which they are? Use that knowledge to answer the questions in the following table, if possible. Important: do not try to figure out exactly which elements are marked, and then use your knowledge of the properties of each specific element. You don't need to. You will also be marked wrong for any answer, correct or not, that can't be determined from the rough location of each marked element in the Periodic Table. Element X Element Y Which element in the gas phase is more likely to glow green or yellow in a flame? X 5 O Can't say without more information. O Element X Which element is more likely to form an ionic compound with chlorine? O Element Y O Can't say without more information. O Element X Which element in the solid state is probably brittle, so that it breaks before bending? Element Y Can't say without more information. Explanation Checkarrow_forward
- consider the atom with the chemical symbol Ru. What would be the charge on an atom of Ru which only has 41 electronsarrow_forwardUse CER, claim, evidence, and reasoning. Claim is atoms of elements in the same group have the same number of valence electrons and charges. Use evidence from the chart and include reasoning.arrow_forwardISOTOPES Isotopes are atoms that have the same atomic number but different mass number. Most elements have two or more isotopes. For example, there are three isotopes of hydrogen: 1H, 2H, ³H. Since these isotopes are all hydrogen element, they should obviously all have the same atomic number of 1, but as you can see they all have different mass numbers. And the reason for having different mass numbers should be due to having different neutron numbers: 'H having zero neutron; 2H one neutron; ³H two neutrons. QUESTION Element mercury has six isotopes: 198Hg, 199H9, 200Hg, 201H9, 202H9, and 204H9. The abundance (frequency) of each of these isotopes is measured to be 10.20%, 16.90%, 23.40%, 13.10%, 29.90%, and 6.500%, respectively. Calculate the average atomic mass for mercury. This result should be very close to the number you can find in the Hg box in the periodic table. Hint: You can solve this question if you carefully exam the solution procedures you already exercised in the previous…arrow_forward
- 8.) A spy is using elemental symbols to write messages. The number that is used is the sumof the atomic number and the highest principal quantum number (n) for the element.Decode the two messages below.a.) 10, 12, 58, 11, 7, 44, 63, 66b.) 9, 99, 30, 95, 19, 47, 79arrow_forward1.) Identify the missing information for each atom or ion. Note that the atoms and ions are not necessarily neutral.A Se ion has a mass number of 76 and a charge of −2 . Determine the number of neutrons, protons, and electrons in this ion.number of neutrons:number of protons:number of electrons:An ion has a mass number of 65, 36 neutrons, and a charge of +1 . Identify the element symbol, and determine the number of protons and electrons in this ion.element symbol: number of protons:number of electrons:An atom or ion has 43 neutrons , 36 protons, and 36 electrons. Identify the element symbol, and determine the mass number and charge for this atom or ion.element symbol: mass number:charge: 2.) Determine the number of electrons lost or gained when each atom forms an ion.Be electrons. Se electrons. I electrons. P electronarrow_forwardAn isotope of element Z has a symbol of Z-390 with a charge of - 1. If the element Z has 197 neutrons, how many electrons does the ion possess? Arrange the following ions in order of increasing radius: Na+, Mg2+, F–, Te2–. Group of answer choices Na+ < Mg2+< Te2- < F– Mg2+ < Na+< F– <Te2– Te2–< F– < Mg2+ < Na+ Na+ < Mg2+< F– <Te2–.arrow_forward
- How many valence electrons does each of the following atoms have? titanium, ? = 22 iodine, ? = 53 radium, ? = 88 manganese, ? = 25arrow_forwardPlease don't provide handwriting solutionarrow_forwardGive detailed Solution...show work..don't give Handwritten answer..don't use Ai for answering thisarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY