
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
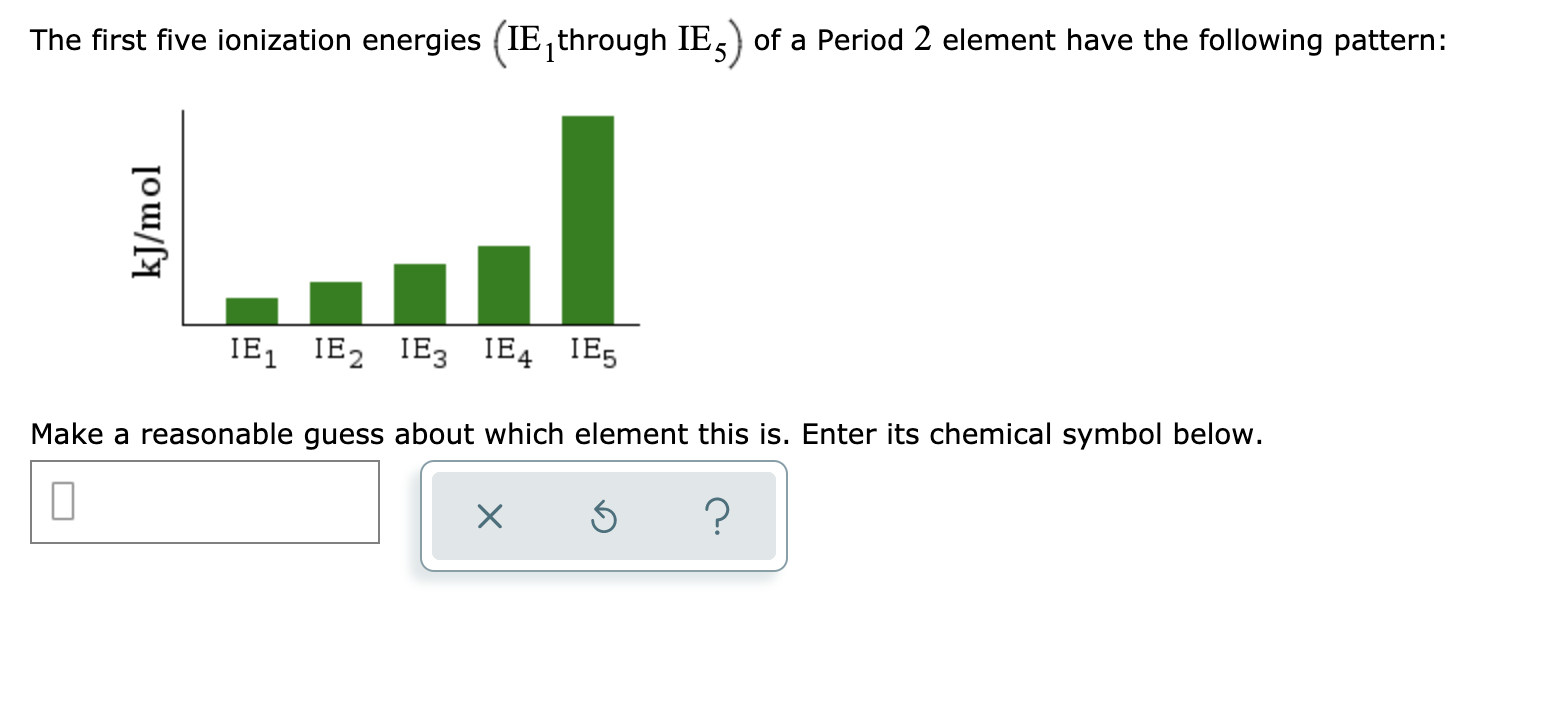
Transcribed Image Text:The first five ionization energies (IE, through IE,) of a Period 2 element have the following pattern:
IE, IE2 IE3 IE4 IE5
Make a reasonable guess about which element this is. Enter its chemical symbol below.
kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Which of the following pairs of atoms and ions are isoelectronic? Li+, Rn S2−, Cl− Fr+, Rn Ra2+, Fr+ I−, Xe As3−, Cl−arrow_forwardIodine (I) would have chemical properties most similar to which of the following elements Te Rb Br Xearrow_forwardConsidering only ions with charges of + 1, + 2, - 1 , and - 2 , or neutral atoms, give the symbols for 4 species that are isoelectronic with Xe.arrow_forward
- Consider an ionic compound, MX, composed of generic metal M and generic, gaseous halogen X. • The enthalpy of formation of MX is AH; = -513 kJ/mol. • The enthalpy of sublimation of M is AHsub = 123 kJ/mol. • The ionization energy of M is IE = 465 kJ/mol. • The electron affinity of X is AHEA = -325 kJ/mol. (Refer to the hint). • The bond energy of X, is BE = 187 kJ/mol. Determine the lattice energy of MX. AHjattice -795.5 kJ/mol Incorrectarrow_forwardPlease don't provide handwriting solutionarrow_forwardWhich of the following elements is the largest? a barium b titanium c rubidium d sodiumarrow_forward
- An atomic anion with a charge of -2 has the following electron configuration: 1s 2s 2p 3s 3p What is the chemical symbol for the ion? W How many electrons does the ion have? How many 2s electrons are in the ion?arrow_forwardwhat is the elemental form of fluorine? F(g) F- (aq) F+(g) or none of the these?arrow_forwardWrite the number of valence electrons in the elements Ca,Si,Kr,S and Naarrow_forward
- When selenium (atomic number 34 on the periodic chart) becomes an ion, with which atom does the ion become isoelectronic? Write the elemental symbol of this atomarrow_forwardWhich of these elements has the highest first ionization energy? silicon sodium chlorine sulfur phosphorusarrow_forwardThe first five ionization energies (IE, through IE,) of a Period 4 element have the following 1 ll IE, IE, IE3 IEĄ IE, Make a reasonable guess about which element this is. Enter its chemical symbol below. kJ/molarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY