
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
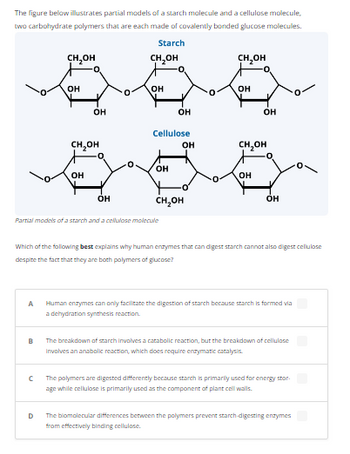
Transcribed Image Text:The figure below illustrates partial models of a starch molecule and a cellulose molecule,
two carbohydrate polymers that are each made of covalently bonded glucose molecules.
Starch
A
B
CH₂OH
C
OH
D
OH
CH₂OH
OH
OH
0
CH₂OH
OH
Partial models of a starch and a cellulose molecule
Cellulose
OH
OH
OH
CH₂OH
0
CH₂OH
OH
Which of the following best explains why human enzymes that can digest starch cannot also digest cellulose
despite the fact that they are both polymers of glucose?
OH
CH₂OH
OH
OH
Human enzymes can only facilitate the digestion of starch because starch is formed via
a dehydration synthesis reaction.
The breakdown of starch involves a catabolic reaction, but the breakdown of cellulose
Involves an anabolic reaction, which does require enzymatic catalysis.
The polymers are digested differently because starch is primarily used for energy stor
age while cellulose is primarily used as the component of plant cell walls.
The biomolecular differences between the polymers prevent starch-digesting enzymes
from effectively binding cellulose.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- look at the molecule form for sucrose why does carbohydrates not follow the one to one carbon hydrogen oxygen ratio of monosaccharidearrow_forwardIdentify this molecule CH₂OH H HO ОН H nucleic acid saturated fat carbohydrate phospholipid amino acid H OH H OHarrow_forwardBased on the given figure, answer the following questions H CH₂OH H OH H O H OH H J H Glucose CH₂OH H OH alpha 1, 6 H H beta 1, 4 alpha 1, 4 O H CH₂OH H OH H OH H OH O H H J 1. o CH₂₂ H OH H H O The monomer of this molecule is? What is the branching point? OH H H J The name of this polymer in plants? The importance of this molecule o The main glycosidic bond in the chain? Sucrose Fructose Galactose CH₂OH H OH O H OH Structural sugar H Harrow_forward
- Chemistry The glucose molecule shown below is numbered 1-6. Show where those numbers would appear in pyruvate and acetyl-CoA (draw the molecules with the associated numbers). Note that each of the carbon atoms on these two molecules will have two of the numbers from glucose associated with them.arrow_forwardsee imagearrow_forwardThe term used to describe a class of carbohydrates capable of transferring electrons to other molecules are: O Disaccharides O Glycosidic Linkages O Reducing Sugars O Glycogenarrow_forward
- You can choose one or more than one option Which of the following compounds can be used to make glucose in animals? BIOCHEMISTRY basic Glycerol Lactate Pyruvate Lysine Valine Which of the following atoms is found in RNA and DNA: MOLECULAR BIOLOGY basic Iron Oxygen Carbon Sulfur Hydrogenarrow_forwardx * x A () lipids: fatty acids and glycerol proteins: amino acids carbohydrates: disaccharides H HỎ D carbohydrates: monosaccharides E nucleic acids: nucleotides CH₂OH H OH H Carbon, hydrogen, and oxygen from sugar molecules may combine with other elements to form other biomolecules. The picture above shows sucrose, a disaccharide used to form larger biomolecules. Biomolecules are all made of smaller units called monomers. Examine the list of biomolecules below. Select ALL of the choices that correctly pair the biomolecule with its monomer. QH H H OH Y CH₂OH 0 Q CH₂OH H HO OH H H 5 of 10 32arrow_forwardWrite a sample chemical reaction of chymotrypsin in a complete balanced equation label the following properly: substrate/s, cosubstrate/s, and cofactor/s Show all the changes in the reacting components small molecules should be in their skeletal form large molecules could be shown as hybrid structures - reacting/interacting groups in skeletal form, the rest of the molecule as abbreviations/blocks/shapesarrow_forward
- Moore's Test We did an experiment about carbohydrate chemistry and the professor did not elaborate on the details. She just gave the positive result and reagent. I would like to ask the following: What type of reaction occurred when the samples (enumerated) reacted with the MOORE'S reagent? Give the chemical equation for each and the principle/mechanism of the reaction. Glucose Sucrose Fructosearrow_forwardThe molecular structure shown here represents which lipid component? monounsaturated fatty acid 18. H2C CCH2 H2C CH2 H2C CCH2 H2C3 saturated fatty acid simple lipid d) trans fatty acid polyunsaturated fatty -H acid H- H2C3 CCH2 H2C. CCH2 H2C CCH2 * H¿C CH3arrow_forwardWhen comparing starch and cellulose, which carbohydrate would be more useful as a component of bacterial cell walls? Explain. Use the following terms as part of your answer: alpha-glucose, beta-glucose, carbons, 3D structurearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education