
Chemistry: Principles and Practice
3rd Edition
ISBN: 9780534420123
Author: Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Aa.67.
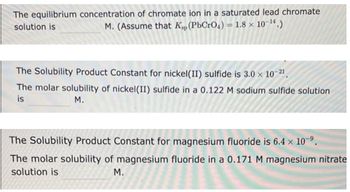
Transcribed Image Text:The equilibrium concentration of chromate ion in a saturated lead chromate
solution is
M. (Assume that Kp (PbCrO4) = 1.8 × 10-¹4.)
The Solubility Product Constant for nickel(II) sulfide is 3.0 × 10-2¹.
The molar solubility of nickel (II) sulfide in a 0.122 M sodium sulfide solution
is
M.
The Solubility Product Constant for magnesium fluoride is 6.4 x 10-⁹.
The molar solubility of magnesium fluoride in a 0.171 M magnesium nitrate
solution is
M.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The ore cinnabar (HgS) is an important source of mercury. Cinnabar is a red solid whose solubility in water is 5.5 X 10-2 mol L-1. Calculate its \p. What is its solubility' in grams per 100 g of water?arrow_forwardUse the solubility product constant from Appendix F to determine whether a precipitate will form if 10.0 mL of 1.0 106 M iron(II) chloride is added to 20.0 mL of 3.0 104 M barium hydroxide.arrow_forward. The solubility product of iron(III) hydroxide is very small: Ksp=41038at 25 °C. A classical method of analysis for unknown samples containing iron is to add NaOH or NH3. This precipitates Fe(OH)3, which can then be filtered and weighed. To demonstrate that the concentration of iron remaining in solution in such a sample is very small, calculate the solubility of Fe(OH)3in moles per liter and in grams per liter.arrow_forward
- The following question is taken from a Chemistry Advanced Placement Examination and is used with the permission of the Educational Testing Service. Solve the following problem: MgF2(s)Mg2+(aq)+2F(aq) In a saturated solution of MgF2 at 18 C, the concentration of Mg2+ is 1.21103M . The equilibrium is represented by the preceding equation. (a) Write the expression for the solubility-product constant, Ksp, and calculate its value at 18 C. (b) Calculate the equilibrium concentration of Mg2+ in 1.000 L of saturated MgF2 solution at 18 C to which 0.100 mol of solid KF has been added. The KF dissolves completely. Assume the volume change is negligible. (c) Predict whether a precipitate of MgF2 will form when 100.0 mL of a 3.00103 -M solution of Mg(NO3)2 is mixed with 200.0 mL of a .2.00103 -M solution of NaF at 18 C. Show the calculations to support your prediction.. (d) At 27 C the concentration of Mg2+ in a saturated solution of MgF2 is 1.17103M . Is the dissolving of MgF2 in water all endothermic or an exothermic process? Give an explanation to support your conclusion.arrow_forwardGiven that the Gf for Pb2+(aq) and Cl-(aq) is -24.3 kJ/mole and -131.2 kJ/mole respectively, determine the solubility product, Ksp, for PbCl2(s).arrow_forwardWrite the expression of the reaction quotient for the ionization of HOCN in water.arrow_forward
- In a particular experiment, the equilibrium constant measured for the reaction, Cl2(g)+NO2(g)Cl2NO2(g), is 2.8. Based on this measurement, calculate AG° for this reaction. Calculate AG° using data from Appendix E at the back of the book and discuss the agreement between your two calculations.arrow_forwardSome barium chloride is added to a solution that contains both K2SO4 (0.050 M) and Na3PO4 (0.020 M). (a) Which begins to precipitate first: the barium sulfate or the barium phosphate? (b) The concentration of the first anion species to precipitate, either the sulfate or phosphate, decreases as the precipitate forms. What is the concentration of the first species when the second begins to precipitate?arrow_forward. Lead(II) chloride, PbCl2(s), dissolves in water to the extent of approximately 3.6102Mat 20 °C. Calculate Kspfor PbCl2(s), and calculate its solubility in grams per liter.arrow_forward
- Because calcium carbonate is a sink for CO32- in a lake, the student in Exercise 12.39 decides to go a step further and examine the equilibrium between carbonate ion and CaCOj. The reaction is Ca2+(aq) + COj2_(aq) ** CaCO,(s) The equilibrium constant for this reaction is 2.1 X 10*. If the initial calcium ion concentration is 0.02 AI and the carbonate concentration is 0.03 AI, what are the equilibrium concentrations of the ions? A student is simulating the carbonic acid—hydrogen carbonate equilibrium in a lake: H2COj(aq) H+(aq) + HCO}‘(aq) K = 4.4 X 10"7 She starts with 0.1000 AI carbonic acid. What are the concentrations of all species at equilibrium?arrow_forwardBecause barium sulfate is opaque to X-rays, it is suspended in water and taken internally to make the gastrointestinal tract visible in an X-ray photograph. Although barium ion is quite toxic, barium sulfate’s /Csp of 1.1 X 10-,<) gives it such low solubility' that it can be safely consumed. What is the molar solubility' of BaSO4. What is its solubility' in grams per 100 g of water?arrow_forwardMagnesite (magnesium carbonate, MgCO3) is a common magnesium mineral. From the solubility product constant, find the solubility of magnesium carbonate in grams per liter of water.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning