Related questions
Concept explainers
Question
Transcribed Image Text:Macmilla
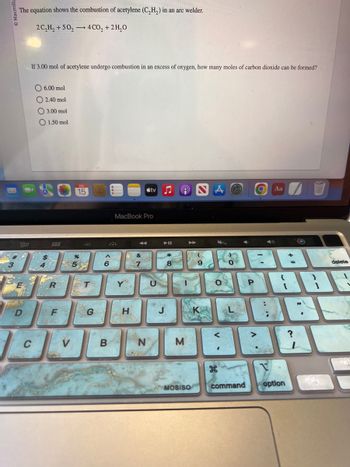
The equation shows the combustion of acetylene (C₂H₂) in an welder.
2 C₂H₂ +50₂ 4 CO₂ + 2H₂O
20
D
If 3.00 mol of acetylene undergo combustion in an excess of oxygen, how many moles of carbon dioxide can be formed?
C
6.00 mol
O2.40 mol
3.00 mol
O 1.50 mol
R
F
V
15
25
%
M
T
h
G
6
B
MacBook Pro
Y
H
&
N
tv
N
U
916
8
ONA
M
▶
MOSISO
AC
O
<
H
P
• V
| '
:
Aa
{
[
command option
+11
?
}
B
delete
Expert Solution
arrow_forward
Step 1
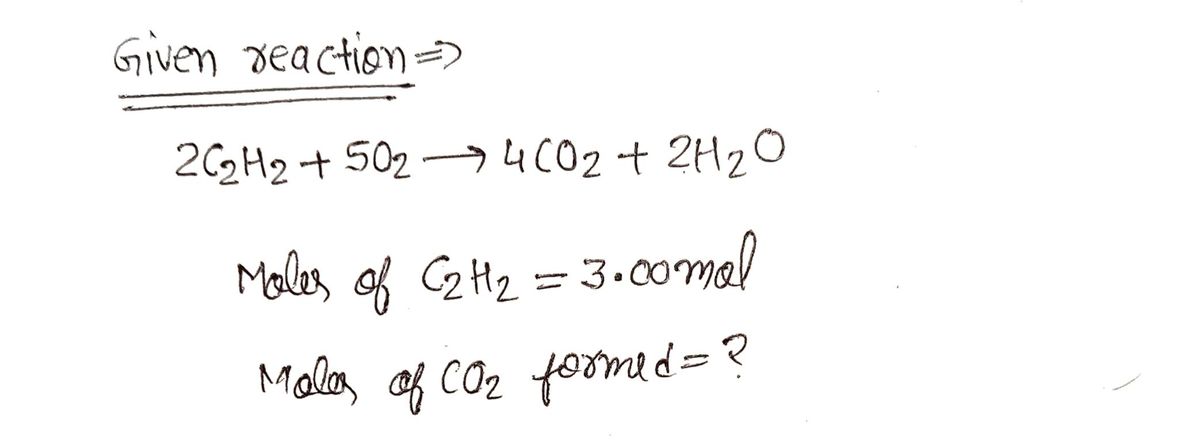
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.