
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
I have no clue on how to set up this problem or solve it. What formula do I use?
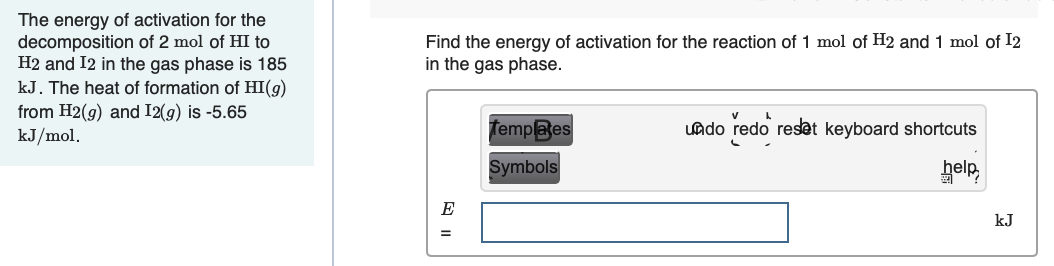
Transcribed Image Text:The energy of activation for the
decomposition of 2 mol of HI to
H2 and I2 in the gas phase is 185
kJ. The heat of formation of HI(g)
from H2(g) and I2(g) is -5.65
kJ/mol.
Find the energy of activation for the reaction of 1 mol of H2 and 1 mol of I2
in the gas phase.
Templaes
uado redo reset keyboard shortcuts
Symbols
help,
kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 15) How many grams would 4.904 x 10^22 atoms of Lithium weigh? Show all work and report your answer to the correct units and significant figures.arrow_forwardWhat kind of molecule can you make by moving Cl = Chlorine and Mg=Magnesium?arrow_forwardA mixture was found to contain 1.05g of SiO2, 0.69 g of cellulose, and 1.82 g of calcium carbonate. What percentage of calcium carbonate is in the mixture? Write the formula of calcium carbonate.arrow_forward
- Platinum is an expensive and rare metal used in catalytic converters. Much research has been devoted to maximizing reactive properties of other less expensive metals by reducing their size to the nanoscale. Smaller particles are more reactive because they have a larger surface area to volume ratio. Which type of atom in a nanoparticle is more reactive? An atom on the surface of a particle An atom in the interior of a particle The location of an atom in the nanoparticle does not influence its reactivity.arrow_forward1. Give reasons why the helium atom is smaller than the cesium atom. 2. A student got a problem wrong. They were asked, “How many grams of MgCl2 (molar mass = 95.21 g/mol) are needed to make 3.16 L of a 0.500 M MgCl2 solution?” The correct answer is 150. but the student got 1.58. What mistake did the student make? 3. To solve the following problem what is the reason you must subtract the vapor pressure of water, 24 torr, from the total pressure, 747 torr in order to solve the problem? “A 500.0-mL sample of H2 gas is collected over water at 298 K and 747 torr. What volume would the dry H2 gas occupy at STP? (Given: the vapor pressure of water at 298K is 24 torr).” By the way, the answer is .436 liters. NOTE: I am not asking you to solve this problem. I am asking the reason for subtracting the vapor pressure. If you simply solve the problem, you will get no points.arrow_forwardYou have a lot of paperwork laying around. A ream of stacked paper is 500 sheets and is 2.0 inches thick. Let's say you had a mole of paper. (1 mole of paper = 6.022 x 10^23 sheets of paper) and it was all stacked up in 10 stacks of equal height, each containing a tenth of a mole of sheets. The ten stacks sit one on top of the other. How high are they?arrow_forward
- If green spheres represent chlorine atoms, yellow-green spheres represent fluorine atoms, white spheres represent hydrogen atoms, and all the molecules are gases, A.write the formula for each of the reactants. Express your answers as chemical formulas separated by a comma B. write the formula for each of the products. Express your answers as chemical formulas separated by a comma.arrow_forward8arrow_forwardSelect all the meso compounds. CI ...C/ Br Brarrow_forward
- You wish to prepare 204 grams of 14.9% (w/w) NaNO3. Assume that the density of water is 1.00 g/mL. You will need grams of sodium nitrate and mL of water.arrow_forwardPlatinum is a precious metal that is used as a catalyst for many important industrial reactions. Platinum can be purified by reacting platinum(IV) bromide solution with aluminium metal. To test this purification process, a student reacted 14.6 kg of aluminium metal with excess platinum(IV) bromide solution. After the reaction was complete, the student had extracted 75.4 kg of platinum metal. From this data, calculate the percent error of this experiment. Hint: Write a balanced chemical reaction equation and calculate the theoretical yield of platinum first. Do not show your work in the space provided. Record only your final answer with the correct number of significant digits and the proper units. Answer: Evaluate your calculated percent error, is this an acceptable percent error?arrow_forward8 Sb (s) + 10 HNO3 (aq) 4 Sb2O5 (s) 5 N20 (2) + 5 H20 (1) If 0.0540 mol of HNO3 reacts, how many g of N20 are formed? Write out the problem on paper showing all conversion factors, unit cancellations, calculations, s.f., etc. Answer the questions related to the setup and calculation for this problem. Be sure to use our periodic table to calculate any molar masses needed (rounded to proper number of decimal places), otherwise your values might be slightly off and answers may be marked as incorrect. Abbreviate units as follows: grams = g, moles = mol. Use the three blanks to enter the number, unit, and substance (in this order) that appears in the denominator of the stoichiometry conversion factor. For the molar mass needed in this problem, use the two blanks to enter the value (rounded to proper dec places) and substance (in this order). g/mol Use the three blanks to enter the number, unit, and substance (in this order) that appears in the numerator of the molar mass conversion factor.…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY