
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
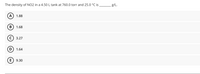
Transcribed Image Text:The density of NO2 in a 4.50L tank at 760.0 torr and 25.0 °C is
g/L.
A
1.88
1.68
С) 3.27
D
1.64
E
9.30
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ethane burns in air to give h2o and o2 . 2c6h6+ 7o2= 4co2+ 6h2o What volume of o2 (L) is required for complete reaction with 6.8 L of c6h6 ? Assume all gases are measured at the same temperature and pressure. Volume = __L What volume of h2o vapor (L) is produced in the complete reaction of 6.8 L of c6h6 ? Assume all gases are measured at the same temperature and pressure. Volume =___ Larrow_forwardThe volume of 2.2 g of NH3 (ammonia) at 27 oC and 570 mm pressure is - Mm of ammonia= 17g/mole * 4.25 L 0.82 L O 3.82 L 1.64 Larrow_forwardA mixture of methane and krypton gases is maintained in a 9.28 L flask at a pressure of 3.79 atm and a temperature of 54 °C. If the gas mixture contains 14.4 grams of methane, the number of grams of krypton in the mixture is ______g. The stopcock connecting a 1.10 L bulb containing carbon dioxide gas at a pressure of 6.01 atm, and a 4.66 L bulb containing helium gas at a pressure of 1.70 atm, is opened and the gases are allowed to mix. Assuming that the temperature remains constant, the final pressure in the system is ______ atm.arrow_forward
- A sample of gas originally at 25.0oC and 700.0 mm Hg of pressure occupies 20.0 liters. What will be the sample's new pressure (in atm) upon heating to 32.3oC and compression to 16.4 liters ? The number of particles remained the same.arrow_forward_10. Propane burns in air according to the equation: C3Hs (g) + 502 (g) → 3CO2 (g) + 4H2O (g) what volume of CO2 would be formed if 5.00 L of propane burns, assuming that all of the gases are under the same conditions? A. 5.00 L B. 15.0 L C. 3.00 L D. 4.00 L E. 1.67L 11 What volume of H would be collected at 22.0°C and a pressure of 713 torr if 2 65 g of zinc reactarrow_forwardAt 150. oC and 3.00 atm, 700. mL of a vapor has a mass of 0.4183 g. What is the molecular weight of the compound? 6.91 g/L 58.0 g/L 62.0 g/L 65.0 g/L 29.1 g/Larrow_forward
- If an experiment's conditions are changed from 15.3 L, 1.7 atm, and 45°C to STP, what will the new volume be? * O 22.5 L O 24.2 L O 22.3 L O 158 Larrow_forwardA sample of methane gas collected at a pressure of 438 mm Hg and a temperature of 292 K has a mass of 11.2 grams. The volume of the sample is____L.arrow_forwardAccording to Dalton, the pressure exerted by a gas inside a container is proportional to the number of molecules of that gas. If 4.00 x 1023 molecules of N2 and 2.00 x 1023 molecules of O2 are mixed in a container they exert a total pressure of 1686 torr. What portion of that pressure (in torr units) is exerted by the N2? Report answer to four significant figures in decimal notation. _____________________ torr, What portion of that pressure (in torr units) is exerted by the O2? Report answer to four significant figures in decimal notation. ____________________ torrarrow_forward
- What is the volume occupied by 15.0 g of nitrogen monoxide gas, NO, at 242K and 690 mm Hg)? 0.432 L 22.4 L 328 L 0.0144 L 10.9 Larrow_forwardCan you help me with the number 6 question? Can you explain step by step including the formula (Gay-Lussac’s Law temperature, pressure)? I need to plug in the fraction to give the correct answer.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY