
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
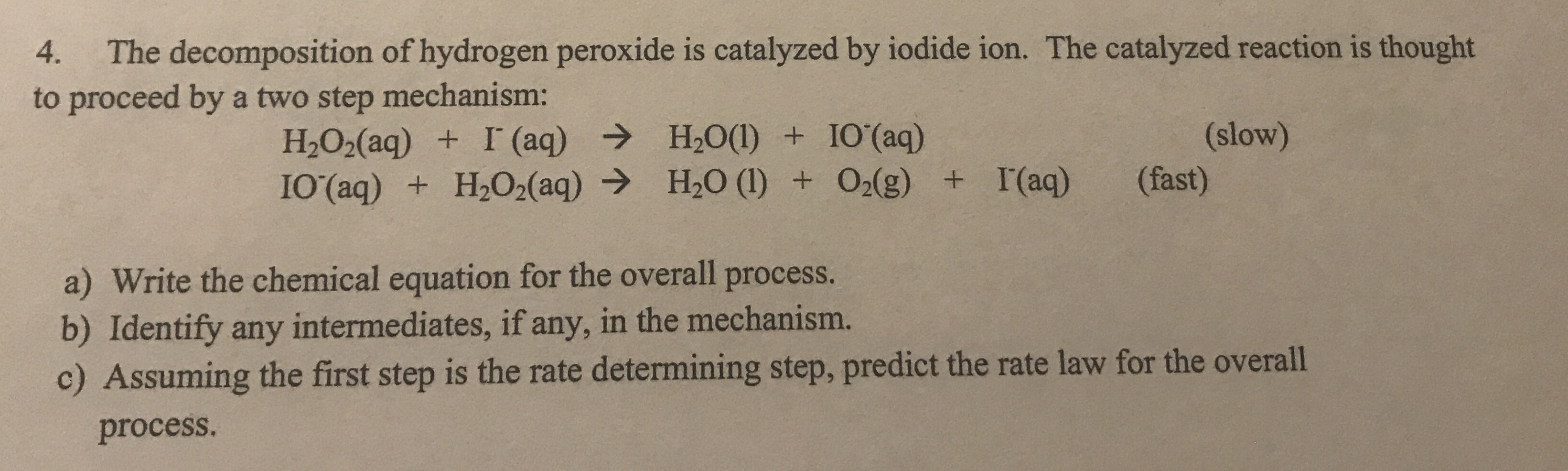
Transcribed Image Text:The decomposition of hydrogen peroxide is catalyzed by iodide ion. The catalyzed reaction is thought
to proceed by a two step mechanism:
4.
H2O2(aq) + I (aq) → H2O(1) + IO'(aq)
I0(aq) + H2O2(aq) → H20 (1) + O2(g) + I(aq)
(slow)
(fast)
a) Write the chemical equation for the overall process.
b) Identify any intermediates, if any, in the mechanism.
c) Assuming the first step is the rate determining step, predict the rate law for the overall
process.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Similar questions
- In the presence of excess thiocyanate ion, SCN-, the following reaction is first order in iron(III) ion, Fe3+; the rate constant is 1.27/s. Fe3+(aq) + SCN-(aq) → Fe(SCN)2+(aq). How many seconds would be required for the initial concentration of Fe3+ to decrease to 12.5% of its initial value? Express your answer to two decijal positions.arrow_forwardConsider the reaction 5B1¯ (aq) + Br0, (aq) + 6H† (aq)→3B12 (aq) + 3H2O(1) The average rate of consumption of Br is 1.16×10-4 M/s over the first two minutes. What is the average rate of formation of Br2 during the same time interval? Express your answer with the appropriate units.arrow_forwardThe reaction 2 NO(g) + O2(g) --> 2 NO2(g) proceeds through the following mechanism: 2 NO(g) --> N2O2(g)N2O2(g) + O2(g) --> 2 NO2(g) (a) The second step of this mechanism is rate-determining (slow). What is the rate law for this reaction? Rate = k [NO] [O2] Rate = k [NO]2 [O2] Rate = k [NO] [O2]2 Rate = k [NO]1/2 [O2] Rate = k [NO] [O2]1/2 Rate = k [NO]2 Rate = k [NO]2 [O2]1/2 (b) What would the rate law be if the first step of this mechanism were rate-determining? Rate = k [NO] [O2] Rate = k [NO]2 [O2] Rate = k [NO] [O2]2 Rate = k [NO]1/2 [O2] Rate = k [NO] [O2]1/2 Rate = k [NO]2 Rate = k [NO]2 [O2]1/2 Rate = k [NO]arrow_forward
- Consider the mechanism. 2 A = B В +С — D Step 1: equilibrium Step 2: slow Overall: 2A +С — D Determine the rate law for the overall reaction, where the overall rate constant is represented as k. rate =arrow_forwardConsider the following proposed reaction mechanism: (1) CIO (aq) + H,O(1)= HCIO(aq) + OH (aq) [fast] (2)I (aq) + HCIO(aq) → HIO(aq) + Cl¯(aq) [slow] (3) OH (aq) + HIO(aq) → H,O() + 10 (aq) [fast] Ignoring water, how many reaction intermediates are present? O 3 O 1 O 2arrow_forwardA. Consider the following proposed mechanism for the production of chlorine dixoide from chlorine and ozone. Draw a reaction coordinate diagram for this reaction. Label reactants, products, transition states, intermediates, and activation energy on your sketch. Assume the reaction is endothermic. NOTE: CI is chlorine, not Carbon lodide Overall: Cl₂ (g) + 203 (g) → 2 CIO₂ (g) + O₂ (g) Step 1: Cl₂ (g) → 2 Cl (g) fast Step 2: Cl (g) + Os (g) CIO₂ +O fast Step 3: O (g) + O3 (g) → 202 (g) fast Step 4: Cl (g) + O2 (g) → CIO2 (g) slowarrow_forward
- Consider the hypothetical reaction A(g) + B(g) C(g) The four containers below represent this reaction being run with different initial amounts of A and B. Assume that the volume of each container is 1.0L. The reaction is second order with respect to A and first order in respect to B. Which of the containers, W,X,Y or Z, would have the greatest reaction rate? Justify your answer.arrow_forwardPeroxynitric acid (HOONO2) is an unstable molecule that decomposes to nitric acid and oxygen: 2HOONO2(aq) → 2HNO3(aq) + O2(g)When the concentration of peroxynitic acid is graphed against time, the resulting plot is curved, but if the logarithm of this concentration is plotted, we instead get a straight line. Based on this, which statement is true? a) This decay is a second order in peroxynitric acid. b) The slope of the straight-line graph is the rate constant. c) One needs the concentration of peroxynitric acid to calculate its half-life. d) The rate law appears to be of the form -Δ[HOONO2]/Δt = k[HOONO2].arrow_forwardThe reaction 2 NO(g) + O2(g) --> 2 NO2(g) proceeds through the following mechanism: 2 NO(g) --> N2O2(g)N2O2(g) + O2(g) --> 2 NO2(g) (a) The second step of this mechanism is rate-determining (slow). What is the rate law for this reaction? Rate = k [NO] [O2] Rate = k [NO]2 [O2] Rate = k [NO] [O2]2 Rate = k [NO]1/2 [O2] Rate = k [NO] [O2]1/2 Rate = k [NO]2 Rate = k [NO]2 [O2]1/2 (b) What would the rate law be if the first step of this mechanism were rate-determining? Rate = k [NO] [O2] Rate = k [NO]2 [O2] Rate = k [NO] [O2]2 Rate = k [NO]1/2 [O2] Rate = k [NO] [O2]1/2 Rate = k [NO]2 Rate = k [NO]2 [O2]1/2 Rate = k [NO]arrow_forward
- Assume that the formation of nitrogen dioxide, 2 NO(g) + 02(g) – 2 NO2(g) is an elementary reaction. (a) Write the rate law for this reaction. (Rate expressions take the general form: rate = k. [A]ª . [B]b.) chemPad О Help Greek - rate=k•[NO]2.[02] rate=k*[NO]^2*[O_2] Correct. (b) A sample of air at a certain temperature is contaminated with 1.9 ppm of NO by volume. Under these conditions, can the rate law be simplified? If so, write the simplified rate law. If not, repeat your answer from above. (Rate expressions take the general form: rate = k . [A]ª . [B]b. Use k' for the new rate constant as needed.) chemPad O Help Greek - rate=k':[NO]2 rate=k*[NO]^2 Correct. (c) Under the conditions described in (b), the half-life of the reaction has been estimated to be 6.7x103 min. What would the half-life be if the initial concentration of NO were 12.4 ppm? 4.0 |1030192 X min Supporting Materials Periodic Table Constants and E Supplemental Dataarrow_forwardConsider the reaction 5Br (aq) + BrO, (aq) + 6H+ (aq)→3B12 (aq) + 3H20(1) The average rate of consumption of Br is 1.36x104 M/s over the first two minutes. What is the average rate of formation of Br2 during the same time interval? Express your answer with the appropriate units. • View Available Hint(s) HẢ |] ? Rate of formation of Br2 Value Units %3Darrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY