
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
Can you answer this ASAP
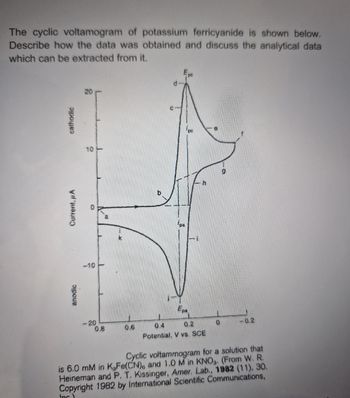
Transcribed Image Text:The cyclic voltamogram of potassium ferricyanide is shown below.
Describe how the data was obtained and discuss the analytical data
which can be extracted from it.
anodic
Current, A
cathodic
-10
0
10
20
k
b
9
h
Epa
-20
0.8
0.6
0.4
0.2
0
-0.2
Potential, V vs. SCE
Cyclic voltammogram for a solution that
is 6.0 mM in K,Fe(CN)6 and 1.0 M in KNO. (From W. R.
Heineman and P. T. Kissinger, Amer. Lab., 1982 (11), 30.
Copyright 1982 by International Scientific Communications,
Inc)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Stripping #1 Ammonia is stripped from a dilute aqueous solution by countercurrent contact with air in a column containing seven sieve trays. The equilibrium relationship is ye = 0.80 xe, and when the molar flow of air is 1.5 times that of the solution, 90% of the ammonia is removed. %3D (a) How many ideal stages does the column have and what is the stage efficiency? (b) Under what thermodynamic conditions (temperature, pressure) is the column operating? [Discuss]arrow_forward14. The conductivity of 0.20 MKC solution at 298 Kis 0.0248 Sam-1. What will be its molar conductivity? (a) 124 Samf (b) 124 cm' (c) 124 ohm' an equiv¹ (d) 124 Sam³ md-¹arrow_forwardThe Nernst-Planck equation (shown below) describes the motion of a charged chemical species in a fluid. dC; z,FC; dv J; = - D; dx RT dx' What are the units for the ion flux J, where: zis the valence state of the ion (unitless) C is the concentration (mol/m³) Fis the Faraday constant (Coulomb/mol) Ris the ideal gas constant (kg m²/(s² mol °K)) Tis the temperature (K) dCi/dx is the concentration gradient (mol/m*) dV/dx is the electric potential gradient (V/m) D; is the diffusion coefficient (m²/s) Note that Coulomb is a unit of charge and V is volts where 1 V= 1 Joule/Coulomb)arrow_forward
- The resistance of a conductivity cell containing a 0.02 M aqueous solution of KCl was fond to be 83 ohms at 298 K. The conductivity of the 0.02 M KCl solution at this temperature is 0.00277 S cm-1. When the cell was filled with a 0.005 M aqueous solution of K2SO4, it exhibited a resistance of 326 ohms at the same temperature. What is the molar conductivity of the potassium sulfate solution? [ answer should be 141 S cm2 mol-1]thank uarrow_forwardTooth enamel consists mainly of the mineral calcium hydroxyapatite, Can(PO)(OH). Trace elements in teeth of archeological specimens provide anthropologists with clues about diet and disease of ancient people. Students at Hamline University measured the trace element strontium in cnamel from extract wisdom teeth by atomic absorption spectroscopy. Solutions were prepared with a constant total volume of 10.0mL. containing 0.750mg dissolved tooth enamel plus variable concentration of added Sr. \table[[Added Sr (ng/mL- ppb), Signal (arbitrary units)], [0, 28.0], [2.50, 34.3], [5.00, 42.8], [7.50, 51.5], [10.00, 58.6]] a) Prepare a graph to find the concentration of Sr in the 10mL sample solution in part per billion = n m L. (2%) b) Find the concentration of Sr in tooth enamel in part per million = μ (3%) 5. Tooth enamel consists mainly of the mineral calcium hydroxyapatite, Ca(PO)(OH). Trace elements in teeth of archeological specimens provide anthropologists with clues about diet and…arrow_forwardAfter correction for the water conductivity, the conductivity of a saturated aqueous solution of AgCI at 25 °C was found to be 0.1887 mS m-1. Given that the limiting molar conductivity of AgCI at this temperature is 138.3 S cm2 mol-1 , what is the solubility of silver chloride at this temperature?arrow_forward
- V5arrow_forwardCalculate the persistency (S) of VX at 25oC ,VX: MW= 267.38; Po = 0.0007 torr @ 25oCarrow_forwardA sample of steel (0.506 g) was digested in acid and the solution made up to 100 cm3 with deionised water. Aliquots (25.0 cm3) of this solution were transferred into two volumetric flasks (100 cm3) labelled A and B. Flask A was made up to volume with deionised water. To flask B was added 10.0 cm3 of a 260 ppm standard solution of Mn2+ which was then also made up to volume with deionised water. The following absorbance results were obtained: Solution A = 0.356, Solution B = 0.578. Use these results to calculate the percentage (% w/w) of manganese in the steel sample.arrow_forward
- A chemist received different mixtures for analysis with the statement that they contained NaOH, NaHCO3, Na2CO3, or compatible mixtures of these substances, together with inert material. From the data given, identify the respective materials, and calculate the percentage of each component. 1.000g samples and 0.2500N HCl were used in all cases. (A) For Sample W: With PP, 24.32ml was used. A duplicate sample required 48.64ml with MO. (B). For Sample X: The addition of PP caused no color change. With MO, 38.47ml of the acid was required. (C). For Sample Y: To cause a color change in the cold with PP, 15.29ml of the acid was necessary, and an additional 33.19ml was required for complete neutralization. (D) For Sample Z: The sample was titrated with acid until the pink of PP disappeared; this process required 39.96ml. On adding an excess of the acid, boiling, and titrating back with alkali, it was found that the alkali was exactly equivalent to the excess acid added.arrow_forwardFor a Copper-Nickel (Cu-Ni) alloy of composition 65 wt % Ni and 35 wt % Cu at 1350 °C (Point B), refer to the phase diagram provided in Figure 2 and answer the following. (1) What is composition of liquid phase? (ii) What is composition of solid phase? (iii) What fraction of the system is liquid for a 65% Ni alloy at 1250 °C? (iv) What fraction of the system is solid for a 65% Ni alloy at 1250 °C? Composition (at% Ni) 20 40 60 80 100 1600 2800 1500 Liquid 1453°C 2600 1400 58 Liquidus line 70 Solidus line 2400 1300 a +L 1200 2200 1100 2000 1085°C 1000 20 40 60 65 80 100 (Cu) Composition (wt% Ni) (Ni) Figure 2. Copper (Cu) and Nickel (Ni) Phase diagram Temperature (°C) Temperature ("F)arrow_forwardThe following activity data of component A have been obtained for liquid A-B solutions at 1500K. XA 0.10 0.0202 0.20 0,0564 0.30 0.1137 0.40 0.1964 0.50 0.3050 (a) Please approve that these data fit the regular solution model. (b).Please calculate G, G, AGmis, a, and ag at X = 0.3 and T = 2000K (on molar basis).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
