
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
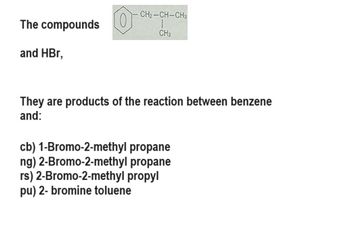
Transcribed Image Text:The compounds O
and HBr,
CH₂-CH-CH3
CH3
They are products of the reaction between benzene
and:
cb) 1-Bromo-2-methyl propane
ng) 2-Bromo-2-methyl propane
rs) 2-Bromo-2-methyl propyl
pu) 2- bromine toluene
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the IUPAC name of the compound shown? CH3 CH3-CH C CH, CH2 CH, CH3 A) 2-methyl-2-propylbutane B) 2-ethyl-2-methylpentane C) 2,2-ethyl-methylpentane D) 3,3-dimethylhexane E) isooctanearrow_forwardWhat is the correct IUPAC name for the following compound? Br 3-bromo-2,3-dimethyl butane 2-bromo-2,3-dimethylbutane 2,3-dimethyl-2-bromobutane O 2,2,3-trimethylbromopropanearrow_forwardFor the following organic structure, identify the type of hydrocarbon derivative and the IUPAC name. OH I CH3-CH-CH-CH₂ CH3 | CH3 BLANK 1: Type of hydrocarbon derivative A/ BLANK 2: IUPAC name of organic compound Aarrow_forward
- What is the correct IUPAC name for the following compound? O A. t-butyl iodide O 1-iodo-1-methyl propane O sec-butyl iodide O 2-iodobutanearrow_forwardWhat is the IUPAC name for the compound? in O 1,5,9-trimethyldecane O 2,5,9-trimethylnonane O 2,5,9-trimethyldecane O 2,6,9-trimethyldodecanearrow_forwardNaming compounds help plsarrow_forward
- Which of the following compounds contains an ether functional group? CH3-CH2- C -CH3 CH3- C-H CH3 CH3- C-O-H CH3 O CH3-CH2-0-CH2- CH3 CH3-O-Harrow_forwardQuestion 21 of 25 Provide the IUPAC name for the compound shown here. da SH 5- 1- 6- 2- 4- 3- di cyclo tert- sec- iso tri proppent benz hex meth eth ene an ane Submit +arrow_forwardWhat is the IUPAC name for the following compound? Select one O 1-propyl-3-isopropyl-2- methylcyclohexane O 1-isopropyl-2-methyl-4- propylcyclohexane 1-isopropyl-3-methyl-4- propylcyclohexane O 4-isopropyl-2-methyl-1- propylcyclohexane 1-propyl-2-methyl-4- Isopropylcyclohexanearrow_forward
- What is the correct IUPAC name of this organic compound? O 4-methyl-3-ethyl-2-heptene O 3-ethyl-4-methyl-2-heptene O 5-ethyl-4-methyl-5-heptene O 3-ethyl-4-methyl-2-heptane O 4-methyl-5-ethyl-5-heptenearrow_forwardWhat is the IUPAC name for the organic compound shown below? O 1,6-dimethylcyclohexene O 1,2-dimethylcyclohexene 2,3-dimethylcyclohexene O 1,2-dimethyl-3-cyclohexenearrow_forwardWhich one of the following compounds does not have a secondary carbon? cydopentane butane 2-mathylbutane propane ethyl chloride C Which one of the following compounds does not have hydrogen bonding? triethylamine ethanol water diethylamine methanol A C What is the correct IUPAC name of the following compound? 3,3-dibromo-2-sec-butoxy-5-chloro-4ethylhexane B) 4,4 dibromo-2-chloro-3-ethyl-5-isobutaxcyhexane Br Br C) 3,3-dibromo-4-(chloroethyl)-2-isobutoxyhexane D) 4,4-dibromo-5-sec-butoxy-2-chloro-3-ethylhexane E) 3,3-dibromo-5-chloro-4-ethyl-2-isobutoxyhexane Rank the following compounds accordingly: " What is the correct order of decreasing ACIDITY of the following compounds? ( -- FCHCHNH HI ICHŁCOH на E strongest>_ >_>_>_weakest -What is the correct order of decreasing BASICITY of the following compounds?; CHSO,Na NaCl CH,CH,NHNa C CHONA CH0O,Na strongest >_>_>_>_weakestarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY