
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Answer the problem of chemical formula step by step ( 4 steps or more) using the three calculations involved in balanced equations:(1) Mole- to- Mole Conversion, (2) Moles- to- Mass Conversion and (3) Mass- to- Mass Calculation.
Problem:
The combustion of propane C3H8, a fuel used in background grills and camp stoves, produces carbon dioxide and water vapor. How many moles of carbon dioxide are formed from 5.60 moles of propane???
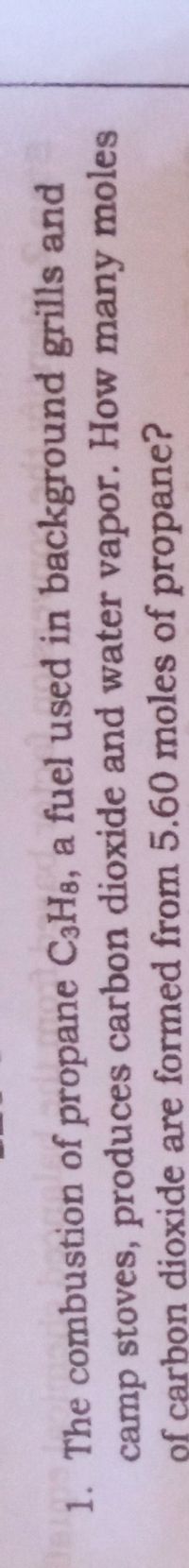
Transcribed Image Text:1. The combustion of propane C3H3, a fuel used in background grills and
camp stoves, produces carbon dioxide and water vapor. How many moles
of carbon dioxide are formed from 5.60 moles of propane?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many ions of Cl-1 would be produced from 7.25x1024 formula units AlCl3? digit _____ (format answer: 1.23x10^23) unit _____ Blank 1: Blank 2:arrow_forwardcalculate the percent yield using the given reaction. We know that there was.0589 mol of bromobenzene, 1004 mol of Mg .289 mol of Et20, 0087 mol of the first product, .0242 mol of NaCIO, ..0044 mol of the final product..arrow_forwardCalculate the number of moles of 2.871 g of lead (11) Carbonatearrow_forward
- 14 of 19 Review I Constants I Periodic Table limiting reactant. Part B How many moles of PCI5 can be produced from 25.0 g of P4 (and excess Cl2)? Express your answer to three significant figures and include the appropriate units. View Available Hint(s) HA ? 202 mol Previous Answers Submit XIncorrect; Try Again; 5 attempts remaining Your answer is the number of moles of P4. Now use this value and the coefficient for PCI5 in the balanced chemical equation to find the number of moles of PCl,. P Pearson F12 II F8 F11 F10 F9 F7 F6 & delete 7 } P U enter II K return ? shift +IIarrow_forwardWhat is the percent yield if a reaction produced 2.25 g of carbon tetrachloride if your theoreticalyield for your experiment was determined to be 2.50 g of carbon tetrachloride.arrow_forwardQuestion 6 (2 points) If you have 4 moles of CH4 molecules, how many atoms do you have? 04 05 20 4 moles O5 moles 20 molesarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY