
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
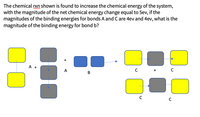
Transcribed Image Text:The chemical rxn shown is found to increase the chemical energy of the system,
with the magnitude of the net chemical energy change equal to 5ev, if the
magnitudes of the binding energies for bonds A and C are 4ev and 4ev, what is the
magnitude of the binding energy for bond b?
+
A +
A
+
C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 6 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The 1995 Nobel Prize in Chemistry was shared by Paul Crutzen, F. Sherwood Rowland, and Mario Molina for their work concerning the formation and decomposition of ozone in the stratosphere. Rowland and Molina hypothesized that chlorofluorocarbons (CFCs) in the stratosphere break down upon exposure to UV radiation, producing chlorine atoms. Chlorine was previously identified as a catalyst in the breakdown of ozone into oxygen gas. Using the enthalpy of reaction for two reactions with ozone, determine the enthalpy of reaction for the reaction of chlorine with ozone. (1)ClO(g)+O3(g)⟶Cl(g)+2O2(g)Δ?∘rxn=−122.8 kJ (2)2O3(g)⟶3O2(g)Δ?∘rxn=−285.3 kJ (3)O3(g)+Cl(g)⟶ClO(g)+O2(g) Δ?∘rxn= ?arrow_forwardEstimate fs0. Assume the energy transfer efficiency (ε) = 0.6 Electron donor: Ethanol Electron accepter: Oxygen Nitrogen Source: Ammoniumarrow_forwardWhich are important thermodynamic parameters associated with the completion of a single step reaction? Explain its energy profile diagram. Sketch diagrams for exo-and endothermic reactions?arrow_forward
- 9:54 AM Mon Aug 9 * 92% AA saplinglearning.com + Sapling Learning SCC 110 Final Exam Audrea Formantes - macmillan learning Sapling Learning > LaGuardia Community College - SCC 110 - Summer21 - TSAI > Activities and Due Dates > sCC 110 Final Exam Check Answer Assignment Score: 74% Resources Hint 01 49 -- Consider the following reaction. MgCl, (aq) + 2 NaOH(aq) → Mg(OH), (s) + 2 NaCI(aq) Calculate the volume of 0.275 M MgCl, that is needed to react completely with 26.2 mL of 0.432 M NaOH. O 41.2 mL O 8.34 mL O 20.6 mL O 82.3 mL !!!arrow_forward9A.2 Write the valence bond wavefunction of the o bond in a C-H group of a molecule.arrow_forward1. Calculate the Coulombic (ionic) interaction energy between atoms for the C=O ∙∙∙H-N hydrogen bond in a peptide at a distance of 1.5Å in units of kJ/mol. Assume that the partial charge on O is −0.434, the partial charge on H is +0.417 and the permittivity of the protein environment is 6. 2. The dipole moment of an individual peptide group is approximately 3.46 D. Assuming that the dipoles line up linearly estimate the energy of interaction (in kJ.mol) of the hydrogen bond in Q1 using a dipole-dipole model.arrow_forward
- 13 Consider the following reaction: H2 (g) + 12 (g) --> 2HI (g) Given the following data: H2: AH° = 0 kJ/mol; S° = 135 J/molK %3D I,: AH°=0 kJ/mol; S° = 111 J/mol K HI: AH° = 22 kJ/mol; S° = 202 J/mol K Determine the AG° in kJ for this reaction at 298K. Do not put units in your answer. Report your answer with two places past the decimal point. Type your answer... Sign of Entropy MacBookarrow_forwardThe reaction between peroxodisulphate (VI) ions, S2O8^2- and iodide ions, I- can be catalyzed by iron(III) ions, Fe^3+. a). Suggest a mechanism for the catalytic reaction b). Sketch energy profile diagram for the catalyzed and uncatalyzed reaction s..ns.arrow_forwardPredict the product for the following reactions. 1. NaOEt Rxn I 2. H20 CH3OH cat. H* Rxn II Rxn I Rxn II но А. OEt OCH3 OCH3 HO "OEt OEt OH С. OCH3 OEt OCH3 D. O., B.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY