
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
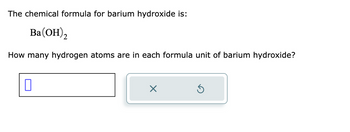
Transcribed Image Text:The chemical formula for barium hydroxide is:
Ba(OH)2
How many hydrogen atoms are in each formula unit of barium hydroxide?
П
×
Ś
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3.97 x 10^24 molecules of NO has what massarrow_forwardDetermine the mass in grams of 5.36 x 10^21 atoms of chromium. (the mass of one mole of chromium is 52.00 g.)arrow_forwardCertain antacids are composed of calcium carbonate, CaCO3. How many grams of calcium are present in a 400 g bottle of CaCO3 ? 235 g 160 g 0.160 g 9.99 garrow_forward
- The molecular formula of rimantadine is C12H21N. How many grams of hydrogen are in 36.01 grams of rimantadine?arrow_forwardWhat is the mass of 8 atoms of copper in grams? 8.44 x 10-22 g 1.33 x 10-23 g 508 g 7.58 x 1022 g 63.5 garrow_forwardWhat is the mass of 1.000 × 1024 Aluminum atoms in gramsarrow_forward
- How many moles of calcium atoms do you have if you have 2.46 × 10²¹ atoms of calcium. (The mass of one mole of calcium is 40.08 g.)arrow_forwardHow many protons, neutrons, and electrons does an atom of sulfur-32 contain? O 15 p, 17 n, 15 e O 16 p, 16 n, 16 e O 16 p, 32 n, 16 e O 32 p, 32 n, 32 e O 16 p, 16 n, 17 e Question 3 Determine the number of moles of aluminum in 59.6 g of aluminum. O 0.453 mol O 2.21 mol O 4.42 mol O 6.65 mol O 1.33 x 1024 molarrow_forwardDetermine the mass in grams of 8.16 x 10^21 atoms of zinc. (The mass of one mole of zinc is 65.39 garrow_forward
- A chemist decomposes samples of several compounds; the masses of their constituent elements are listed. Calculate the empirical formula for each compound. Express your answer as a chemical formula 0.2907 g F, 0.1224 g O 0.524 g Na, 0.569 g As, 0.486 g O 1.443 g Se, 2.591 g Clarrow_forwardHow many grams are in 2.23 x 1024 molecules of NiCl2? And How many grams are in 4.29 x 1023 atoms of platinum?arrow_forwardHow many total ions are in 3.36 g of zinc bromate? O 1.72 x 1022 jons O 6.30 x 1021 ions O 2.09 x 1022 jons O 1.89 x 1022 ionsarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY