
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
7
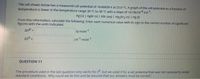
Transcribed Image Text:The cell shown below has a measured cell potential of +0.06839 V at 25.0 °C. A graph of the cell potential as a function of
temperature is linear in the temperature range 20 °C to 30 °C with a slope of +4.18x104 v-K.
Ag (s) | AgBr (5) | KBr (aq) | Hg2Br2 (s) | Hg ()
From this information, calculate the following. Enter each numerical value with its sign to the correct number of significant
figures with the units indicated.
kj-mole 1
As0
JK1.mole"
QUESTION 11
The procedure used in the last question only works for E, but we used it for a cell potential that was not necessarily under
standard conditions. Why could we do this and be assured that our answers must be correct?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following lists the volumes in increasing order (smallest to largest) A) 5ml, 45dl, 2l, 3kl B) 4cl, 6ml, 8l, 10kl C) 19l, 453ml, 22dl, 4clarrow_forwardWhich statement about detecting temperature is TRUE? Nociceptors detect small changes in temperature. Your skin has an equal distribution of temperature receptors. There are separate receptors for hot and cold. There are over 20 types of temperature receptors.arrow_forwardstudent found a bottle of unknown origin in the basement of an "CAUTION—contains С₁H₁O.” No other A old house. The bottle was labelled information was given, but inside the bottle were some white crystals. The student took the bottle to a chemistry laboratory, where she analyzed the crystals with the help of the laboratory staff. C₁2H₁6O reacted with Br₂ in CCl4 adding one mole of Br, and forming C₁₂H₁OB2₂. When a sample of C₁₂H₂O was reacted with O3 followed by Zn/H₂O, two different samples, J and K, were obtained. Compound J had a molecular formula of C.HgO₂, and its NMR is shown below. Compound K had a molecular formula of C₂H₂O, and it could be oxidized to compound L, with a molecular formula of C₂H₂O₂. The IR spectrum of L showed a very wide, strong band centred around 3000 cm*¹. The NMR spectrum of L showed only two different absorptions: a doublet (6H) at 0.9 ppm and a septet (1H) at 3.6 ppm. There was also a singlet (1H) off the scale at 11.8 ppm. students, deduce possible…arrow_forward
- how did you get 0.693arrow_forwardPotentially Useful Information Spectrochemical Series: I < Br < SCN° < Cl° < NO3¯ < F < OH° < C2O4²-~ H2O < NCS < NH3 < en < PPH3 A student has three test tubes containing a metal (M) nitrate solution M(NO3)2 (aq) (where "M" represents a generic transition metal). The student adds aqueous ammonia (NH3) to one test tube, aqueous hydrochloric acid (HCI) to the second tube, and nothing more to the third tube, but forgets to label the tubes. After this, one test tube contains a red solution, one an orange solution, and one a yellow solution. Note: This metal, M, would follow the same rules that we used for Cu(II), Ni(II), and Co(II). The Jahn-Teller Effect does not apply. Complete each statement or answer each question below about the solutions in the three test tubes. The coordination complex causing the orange solution causes a larger splitting between the d orbitals of the metal then the coordination complex causing the... O red solution. O yellow solution. What color light does the…arrow_forward2. CHEMISTRY SUBJECT. You can expect a APPROVE RATING from me Ma'am. I promisearrow_forward
- b Ti2+ [Ar] ↑ 1 3d 45 [Ar] 个 3d 45 [Ar] ↑ ↑ ↑ ↑ За 45arrow_forwardestion 15 of 41 A liquid solvent is added to a flask containing an insoluble solid. The total volume of the solid and liquid together is 75.0 mL. The liquid solvent has a mass of 40.5 g and a density of 0.865 g/mL. Determine the mass of the solid given its density is 2.25 g/mL. mass: 1037 10arrow_forwardRead the buretarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY