
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Transcribed Image Text:133
107
ethyl ether
ethyl alcohol
80
53
一
water
1.
27
ethylene glycol
20
40
60
80
100
120
Temperature (°C)
Pressure (kPa)

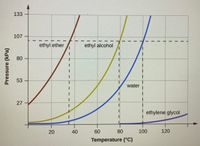
Transcribed Image Text:The boiling point of ethyl ether was measured to be 10 °C at a base camp on the slopes of Mount Everest. Use the graph
above to determine the approximate atmospheric pressure at the camp.
kPa
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For many purposes we can treat butane (C,H10) as an ideal gas at temperatures above its boiling point of – 1. °C. 3 Suppose the pressure on a 8.0 m° sample of butane gas at 11.0°C is reduced to one-third its initial value. O yes Is it possible to change the temperature of the butane at the same time such that the volume of the gas doesn't change? olo O no If you answered yes, calculate the new temperature of the gas. Round your answer to the nearest °C. Explanation Check © 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forwardImagine that a chemist puts 4.33 mol each of C3H8 and O₂ in a 1.00-L container at constant temperature of 505 °C. This reaction occurs: C3H8(g) + 502(g) 3C0₂(g) + 4H₂O(g) When equilibrium is reached, 0.645 mol of CO₂ is in the container. Find the value of Keq for the reaction.arrow_forwardFor many purposes we can treat ammonia (NH3) a as an ideal gas at temperatures above its boiling point of -33. °C. 3 Suppose the pressure on a 1.0 m sample of ammonia gas at -2.00°C is reduced to one-third its initial value. Is it possible to change the temperature of the ammonia at the same time such that the volume of the gas doesn't change? If you answered yes, calculate the new temperature of the gas. Round your answer to the nearest °C. yes no °C x10 Sarrow_forward
- A balloon has a volume of 4.7 L when sitting inside a warm building at 22.°C. When the balloon is taken outside to a temperature of 3.3°C, and the air inside the balloon is allowed to come to equilibrium with the outside air, what will be the volume of the balloon? Answer in L, assuming that the pressure in the balloon is 1 atm, both inside the building and outside in the cold air.arrow_forwardConsider the melting points of the substances below (see figures for two compounds). Explain the trend in melting point using your knowledge of intermolecular forces. (In the structures below, carbon is black, hydrogen is white, and oxygen is red.) Substance Melting Point (°C) Molar Mass (g/mol) Cl2 −102 71 Ethyl formate (CH3CH2OCHO) −80 74 Propionic acid (CH3CH2COOH) −20 74 Br2 −7.2 160arrow_forwardSpace probes to Mars have shown that its atmosphere consists mostly of carbon dioxide. The average temperature on the surface of Mars is –55°C with a pressure of 0.00634 atm. Compare the density of CO2 on Mars’s surface with that on the Earth’s surface at 20°C and one atmosphere. (density on mars/density on earth=blank/1)arrow_forward
- A 1.00 LL flask is filled with 1.20 gg of argon at 25 ∘C∘C. A sample of ethane vapor is added to the same flask until the total pressure is 1.350 atmatm . part A: What is the partial pressure of argon, PArPAr Part B: What is the partial pressure of ethane, PethanePethanearrow_forwardConsider the following samples of gas: sample composition pressure temperature A 2.0mol Xe (g) 1.7atm 228.°C B 2.0mol Xe (g) 1.4atm 258.°C C 2.0mol Xe (g) 1.6atm 280.°C sample composition pressure temperature D 1.5mol He (g) 1.5atm 41.°C E 1.5mol Ar (g) 2.4atm 41.°C F 1.5mol Ne (g) 1.2atm 41.°C Draw the set of graphs below that show the distributions of the speed of the atoms in each sample.arrow_forwardHow many joules of heat are required to heat 25.2 g of ethyl alcohol from the prevailing room temperature, 22.5 oC, to its boiling point, 78.5 oC?arrow_forward
- Four samples of the same unknown gas G are listed in the table below. Rank these samples in increasing order of ideality. That is, select 1 next to the sample of G that will behave least like an ideal gas, and select 4 next to the sample of G that will behave most like an ideal gas. How ideal the sample is: pressure volume temperature sample (atm) (L) (°C) А 30.0 6.0 10.0 Choose one) В 30.0 4.0 0.0 (Choose one) 30.0 2.0 - 10.0 (Choose one) ▼ D 20.0 4.0 10.0 |(Choose one)arrow_forwardUse your text or other reference to find the partial pressure of water at 25°C, 26°C, and 27°C and record those values.arrow_forwardThe air pollutant NO is produced in automobile engines from the high-temperature reaction: N₂(g) + O2(g) = 2 NO(g) K 1.7 x 103 at 2300 K.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY