
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:pnc.com/women
b and Mastering
I Course Home
G Which atom's valence ele
course.html?courseld=16985674&OpenVellumHMAC=Deefedfd559048ad75799f5abe16e1aff#10001
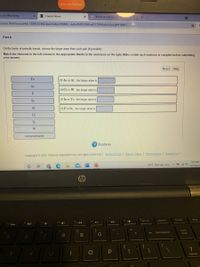
Part A
On the basis of periodic trends, choose the larger atom from each pair (if possible):
Match the elements in the left column to the appropriate blanks in the sentences on the right. Make certain each sentence is complete before submitting
your answer.
Reset Help
Te
Of As or At, the larger atom is
As
Of Cr or W, the larger atom is
F
Of In or Te, the larger atom is
Se
At
Of F or Se , the larger atom is
Cr
In
not predictable
P Pearson
Copyright © 2021 Pearson Education Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions | Contact Us |
8:07 PM
W
80°F Raining now
10/7/2021
prt sc
delete
home
end
10
num
backspace
lock
7.
9.
7.
home
立
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Pick the correct representation of the valence shell for the element with Z 24. 11 a. ns (n-1)d b. ns (n-1)d C. ns (n-1)d d. 11 ns (n-1)d е. ns (n-1)d f. ns (n-1)d g. ns (n-1)d Select one: Oa. O b. Oc. O d. O e. f.arrow_forwardSi What elements are represented by each of the following electron configurations? b) isas°apba3pb d) CAI H6 -arrow_forwardarrow_forward
- The Periodic Table of Elements ETT 10 Down Acrest it gives the element's identity S. faund outside the atom's nucleus an element with an atomie number an electron in the outermost energy level 3. known as the A Family in the periodic table of elements 4 known as the B Family in the periodic table of elements . also known as a group of elementa in the periodic table 7. known as horizontal rows in the periodie table of elements an element with a chemical symbal H 10. 3. same as the atomic numberarrow_forwardPart A Which of the following statements best explains this observation? O The group 4A elements have unusually large atomic radi. O The group 4A elements have much higher first ionization energies than their neighbors in groups 3A and 5A. O The group 4A elements are easier to vaporize than are the group 3A and 5A elements. O The addition of an electron to a group 4A element leads to a half-filled np outer electron configuration. Submit Request Answer Provide Feedbackarrow_forwardWhich drawing best represents the atomic model? A, B, C or D?arrow_forward
- Why does ionization energy in crease across a period? a greater number of electrons? b. Greater number of protons? c. Less electrons? d. Less protons?arrow_forwardQUESTION 1 of an The electron configuration element after it acquires a charge of -1 is 1s22s22p63s23p6 . The element is а. О b. Ar Oc. K )d. Clarrow_forwardMatch the elements to their correct electron configuration: A: [Kr] 4d³ 5s² B: [Ne] 3s² 3p5 C: [Kr] 4d8 5s² D: [Xe] 4f14 5d7 6s² CI Ir Nb Pd [Choose] Choose [Choose] [Choose] Larrow_forward
- Enter the ground state electron configuration on the right of each element listed below (you may use the preceding Noble gas core):arrow_forward1, eight electrons with n = = 2, A neutral atom has two electrons with n = and seven electrons with n = 3. Assuming this element is in its ground state, supply the following information: a. atomic number Atomic number = b. total number of s electrons s electron(s) c. total number of p electrons p electron(s) d. total number of d electrons d electron(s) e. Is the element a metal, metalloid, or nonmetal? metal O metalloid nonmetalarrow_forwardFor the abbreviated electron configuration for the element with 48 protons, which noble gas would you use as a start?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY