
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Using the table in the picture:
The average rate of disappearance of A between 20 s and 40 s i is how many mol/s?
a) 8.5 x 10^-4
b) 1.7 x 10^-3
c) 590
d) 7.1 x 10^-3
e) 1.4 x 10^-3
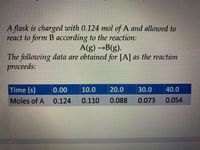
Transcribed Image Text:A flask is charged with 0.124 mol of A and allowed to
react to form B ассоraing to the reaction:
A(g) →B(g).
The following data are obtained forA| as the reaction
proceeds:
Time (s)
0.00
10.0
20.0
30.0
40.0
Moles of A 0.124
0.110
0.088
0.073
0.054
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What fraction of a sample of ³H will be left after 36.78 y?arrow_forward11. Hydrogen peroxide decomposes to form oxygen gas and water. The rate of the reaction was studied at a particular temperature. The following data was obtained, what was the average rate of decomposition'? Initial Concentration (H,O,) Final Concentration (H,O,) Time Elapsed 0.200 mol/L 0.154 mol/L 20 s (3 rarrow_forwardPlease explain and give the correct ansarrow_forward
- How long would it take in minutes, for the amount of potassium -44 to decrease from 80.0 to 10.0mgarrow_forwardA gas sample of Br2 and H2 is irradiated with light with a wavelength range between 440 and 500 nm. It is known that this radiation has sufficient energy to obtain HBr. With this data you can say:a) All the molecules present will give rise to HBr, because the wavelengths are appropriate to activate all the reactants.b) The rate of HBr formation depends directly on the concentration of H2 in the sample.c) The rate of HBr formation depends directly on the concentration of Br2 in the sample.d) If the sample is irradiated with a wavelength of 580 nm, there is no guarantee that HBr will be obtained.arrow_forwardIt is the lab of Decomposition of Hydrogen Peroxide.In this experiment, you will initially determine the initial reaction rate in units of kPa/s. Show how you would convert the initial reaction rate to mol/L - sarrow_forward
- Pls help ASAParrow_forwardFallout from nuclear weapons tests in the atmosphere is mainly 90Sr and 137Cs , which have 28.6- and 32.2-y half-lives, respectively. Atmospheric tests were terminated in most countries in 1963, although China only did so in 1980. It has been found that environmental activities of these two isotopes are decreasing faster than their half-lives. Why might this be?arrow_forwardRadioactive materials are often used in biological studies. A radiation biologist studies the rate of decomposition of a certain substance and obtains the following data: Time (days) 0 4.0 8.0 12.0 16.0 Mass (μg) 26.20 18.13 12.55 8.69 6.01 How many micrograms will remain after 26 days?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY