
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
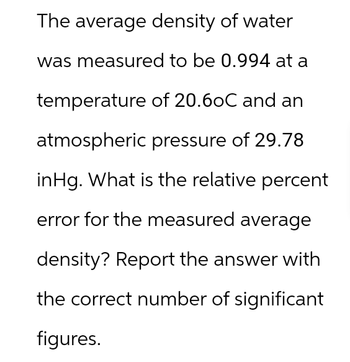
Transcribed Image Text:The average density of water
was measured to be 0.994 at a
temperature of 20.6oC and an
atmospheric pressure of 29.78
inHg. What is the relative percent
error for the measured average
density? Report the answer with
the correct number of significant
figures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A balloon filled with helium has a volume of 5.0 x 10' L at 25°C. What volume will the balloon occupy at 50°C if the pressure surrounding the balloon remains constant? Be sure to answer all parts. Enter your answer in scientific notation.arrow_forwarda balloon at 30 degrees Celcius has a volume of 222ml. if the temperature is increased to 62.1 Celcius and the pressure remains constant, what will be the new volume in ml.arrow_forwardA sample of helium gas was collected over water. The volume of gas was 10.54 L, room pressure was 103.9 kPa, and the temperature was 24.0°C. What was the mass of the helium gas?arrow_forward
- The barometric pressure is 750.8 torr. The water vapor pressure at the temperature at which the experiment is conducted is 21.711 torr. Determine the partial pressure of the gas in torr. Give your answer to the correct number of significant figures.arrow_forwardround your answer to the correct number of significant figures.arrow_forwardA 10.0 gg sample of krypton has a temperature of 25∘C∘C at 567 mmHgmmHg. What is the volume, in milliliters, of the krypton gas? Express your answer to three significant figures and include the appropriate units.arrow_forward
- A balloon at 30.0°C has a volume of 222 mL. If the temperature is increased to 54.1°C and the pressure remains constant, what will the new volume be, in mL?arrow_forwardThe volume of a sample of oxygen is 0.374 mL when the pressure is 1.18 atm and the temperature is 27 ºC. At what temperature (in degrees C) is the volume 1.29 mL and the pressure 0.488 atm? Enter degrees for your unit.arrow_forwardIf a gas cylinder with a volume of 22.00 L contains 19.8 moles of argon gas at 298 K, what is the pressure of the gas in the cylinder in atmospheres? Express the answer to 3 significant figures.arrow_forward
- A balloon at30.0C has a volume of 222 mL. If the temperature is increased to 53.1C and the pressure remains constant, what will the new volume be, in mL?arrow_forwardAccording to Dalton, the pressure exerted by a gas inside a container is proportional to the number of molecules of that gas. If 4.00 x 1023 molecules of N2 and 2.00 x 1023 molecules of O2 are mixed in a container they exert a total pressure of 1686 torr. What portion of that pressure (in torr units) is exerted by the N2? Report answer to four significant figures in decimal notation. _____________________ torr, What portion of that pressure (in torr units) is exerted by the O2? Report answer to four significant figures in decimal notation. ____________________ torrarrow_forwardA sample of argon initially has a volume of 4 L at 25°C. What is the new volume if the temperature was to change to 5°C?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY