
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
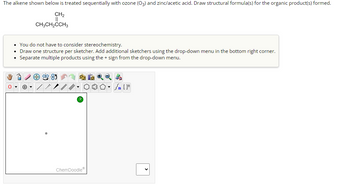
Transcribed Image Text:## Ozonolysis of Alkenes: Reaction with Ozone and Zinc/Acetic Acid
### Description:
The alkene shown is treated sequentially with ozone (O₃) followed by zinc and acetic acid to form organic products. The task is to draw the structural formula(s) of the products formed in this reaction.
### Alkene Structure:
- The starting alkene is represented as follows:
- CH₃CH₂C= C(CH₃)₂
### Instructions:
- **Stereochemistry Consideration**: You do not need to consider stereochemistry for this reaction.
- **Drawing the Structures**:
- Use one sketcher per structure.
- Add additional sketchers if necessary by accessing the drop-down menu in the bottom right corner.
- Separate multiple products using the ‘+’ sign, available in the drop-down menu.
### Sketching Tool:
- A ChemDoodle sketching interface is provided, where you can draw chemical structures using various tools.
### Key Points:
- **Ozonolysis**: This reaction involves cleaving the carbon-carbon double bond in the alkene and forming two carbonyl compounds.
- **Reagents**: Ozone is used to break the double bond, and zinc/acetic acid is utilized to reduce and stabilize the ozonide intermediate to produce final aldehydes or ketones.
Use the provided guidelines and tools to draw the resultant compounds from the described alkene reaction, ensuring accurate representation of chemical structures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Need help!arrow_forwardprovide the IUPAC name of the starting material needed to form the following productsarrow_forwardDraw a structural formula for the substitution product of the reaction shown below. H3C CI CH3OH • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If more than one stereoisomer of product is formed, draw both. • Separate multiple products using the + sign from the drop-down menu.arrow_forward
- Draw the predominant product(s) of the following reactions including stereochemistry when it is appropriate. CH3CH₂-CEC-H + ● ● ● 1 HI Consider E/Z stereochemistry of alkenes. Do not show stereochemistry in other cases. If no reaction occurs, draw the organic starting material. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu.arrow_forwardDraw the structure of the major organic product(s) of the reaction.arrow_forwardQuestion 2 Which statement is consistent with the Hammond postulate? O the transition state of an endothermic reaction step structurally resembles the reactant. O it is used to assign the E/Z stereochemistry of alkenes. the less stable carbocation intermediate is formed in electrophilic additions to alkenes. O in additions of HX to alkenes, the H adds to the less substituted alkene carbon and the X adds to the more substituted alkene carbon. O the transition state of an exothermic reaction step structurally resembles the reactant. A Moving to another question will save this response. 80 F3 54 $ 4 000 000 F4 % 5 F5 6 MacBook Air F6 & 7 ao F7 8 DII F8 ( 9 F9arrow_forward
- References H3C H3C CH3 CH3 CH3 Br DMSO (solvent) CH3 Functional groups such as alkynes react the same in complex molecules as they do in simpler structures. Draw the expected product(s) for this reaction. • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If the reaction produces a racemic mixture, draw both stereoisomers. Separate structures with + signs from the drop-down menu. • Should you want to restart the exercise, the drop-down menu labeled= starting points = can be used to redraw the starting molecule on the sketcher. C opy aste CH3 CH3 CH3 Br CH; 82%arrow_forwardDraw the structure of the major organic product(s) of the reaction. CH3 1. DIBAH, toluene + 2. H30* ChemDoodleⓇ DIBAH diisobutylaluminum hydride, [(CH3)2CHCH₂)2AIH • You do not have to consider stereochemistry. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. D. n [F Previousarrow_forwardThe alkene shown below is treated sequentially with ozone (03) and zinc/acetic acid. Draw structural formula(s) for the organic product(s) formed. CH3 CH3CHCHCH=CH₂ CH3 0 • You do not have to consider stereochemistry. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. ● ▾ 90-85 ChemDoodle - [] laarrow_forward
- NS Predicting the reactants or products of alkene hydration Give one possibility for the mystery reactant R in this organic reaction: OH H+ R+H20 Specifically, in the drawing area below draw the skeletal structure of what R might be. There may be more than one reasonable answer. c+ IIarrow_forwardDraw the reactant(s) for the following reaction.arrow_forwardDraw the structure of the major organic product(s) of the reaction. NC LCI (CH₂=CH)₂CuLi ether, -78° C • You do not have to consider stereochemistry. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right (+) corner. • Separate multiple products using the + sign from the drop-down menu. -85 []arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY