
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
The alcohol below was synthesized via a Grignard reaction. There are TWO different combinations of the Grignard reagent and carbonyl that can be used to make the product. Think about TWO ways to make the product, listing the carbonyl and Grignard reagent in the designated boxes.
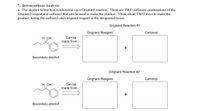
Transcribed Image Text:**Retrosynthesis Analysis:**
The following exercise explores the synthesis of a secondary alcohol via a Grignard reaction. The goal is to identify two distinct combinations of Grignard reagents and carbonyl compounds that can yield the secondary alcohol displayed.
**Molecular Structure:**
- The molecule in question is a secondary alcohol with a phenyl group and a one-carbon chain connected to the alcohol group.
**Grignard Reaction #1:**
- Think about the Grignard reagent and carbonyl compound combination that will produce the secondary alcohol. Complete the provided boxes with the appropriate chemical structures.
**Grignard Reaction #2:**
- Consider an alternative set of Grignard reagent and carbonyl compound that can also synthesize the secondary alcohol. Fill in the designated boxes with the correct structures.
In both pathways, the target is to synthesize the same final product using different starting materials, highlighting the versatility of Grignard reactions in organic synthesis.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- #24 helparrow_forwardb. A chemist wants to use a Grignard reaction to make 1-phenylethanol. Which of the following reactions will successfully form the product? Assume a final acidic workup a each. For any reactions that will not make the product correctly, propose an alternate way to get to the product. Reaction 1 Reaction 2 CH₂Br Br Mg ether OH Mg ether H Harrow_forwardDraw the structure of the product of the reaction below. H₂NNH, NaOH heat Click and drag to start drawing a structure.arrow_forward
- 10. Consider the below reaction between the acetylide ion and methanol. Draw the conjugate acid that is formed as a product in this reaction.arrow_forwardPlease explain it in simple terms for someone who’s confusedarrow_forwardDraw the major product of this reaction. Ignore inorganic byproducts. I I I I I I 1. Hg(Cl)2, CH3OH 2. NaBH4, NaOH Select to Edit OH Please select a drawing or reagent from the question areaarrow_forward
- Specify both the alcohol starting material and the reagents you would use in each step in a synthesis of the compound shown. If the synthesis requires only two steps enter "none" for step 3. r Alcohol Starting Materials 1. methanol 2. ethanol 3. 1-propanol 4. 2-propanol 5. cyclohexanol Reagents available a. LiAlH₁ f. PBr3 b. H₂SO4 c. HCI d. HBr i. CH3 MgBr; then H3O+ e. SOC1₂ j. CH3 CH₂ MgBr; then H3O+ g. CrO3, H₂ SO4, H₂O h. NaH k. CH3 CH₂ CH₂ MgBr; then H3O+ I. C6H5 MgBr (phenylmagnesium bromide); then H3O+ m. (CH3)2 CHMgBr: then H3O+t n. Dess-Martin periodinane (DMP) Write the number/letters of the alchol/reagents in the boxes below. Alcohol starting material Reagent for step 1 Reagent for step 2 Reagent for step 3arrow_forwardDraw the structure of the organic product of the following transformation. Br cat. ACOH NH2arrow_forwardDraw the major product to each of the following reactions.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY