
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Topic Video
Question
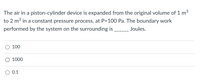
Transcribed Image Text:The air in a piston-cylinder device is expanded from the original volume of 1 m3
to 2 m3 in a constant pressure process, at P=100 Pa. The boundary work
performed by the system on the surrounding is
---_ Joules.
100
O 1000
0.1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Determine the work done by the expanding gas using the trapezoidal rule. from the data in the imagearrow_forwardWater, assumed to be a perfect liquid, has a density of 1000 kg/m3 and a specific heatof 4184 J/kgK. The water undergoes an adiabatic, frictionless process in which itspressure is raised from 170 kPa to 850 kPa. Find the following:(a) the change of specific internal energy (J/kg)(b) the change in temperature (K)arrow_forwardQ4\closed system of 2kg undergoes adiabatic process. The work done on the system is 30000 J. if the relation velocity during the process is (V2² - V12= 216 ) the elevation of system increase from 32M to 77M. Determine the change in internal energy of the systemarrow_forward
- A closed system of constant volume experiences a temperature rise of 20 ⁰C when a certainprocess occurs. The heat transferred in the process is 18 kJ. The specific heat at constantvolume for the pure substance is 1.2 kJkg-1K-1, and the system contains 2 kg of this substance.Determine the change in internal energy and the work done.arrow_forwardRefrigerant 134a expands in a piston-cylinder assembly from p₁= 200 lb/in² and T₁ = 140°F to p₂ = 30 lb/in² and T₂ = 80 °F. The mass of refrigerant is 0.46 lb. During the process, the work done by the refrigerant is 4.32 Btu. Kinetic and potential energy effects are negligible. Determine the initial volume of the refrigerant, V₁, in ft³, and the heat transfer for the process, in Btu. Step 1 * Your answer is incorrect. Determine the initial volume of the refrigerant, V₁, in ft³. V₁= 0.105743 ft3arrow_forwardCalculate the work associated with a system when its volume is compressed from 81.6 L to 30.9 L against a constant pressure of 1.23 bar. Express your final answer in J and 2 significant figuresarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY