
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
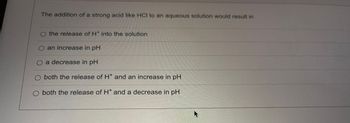
Transcribed Image Text:The addition of a strong acid like HCI to an aqueous solution would result in
O the release of H* into the solution
an increase in pH
O a decrease in pH
O both the release of H* and an increase in pH
O both the release of H* and a decrease in pH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What does a buffer solution do?* increase the pH neutralizes excess hydrogen and hydroxyl ions decreases the pH maintains an almost constant pH A solution is composed of 30 mL of 2.5 M H2SO4 and 25 mL of 6.4 N KOH. The resulting solution s* Oacidic O neutral and ionic basic non-ionicarrow_forwardWhat is the pH and percent ionization in a 0.145 M solution of sulfurous acid?arrow_forward9. Which types of solutions contain equal [H30*] and [OH ]? Acidic solutions Basic solutions Neutral solutions O Ionic solutionsarrow_forward
- 28arrow_forwardWhich is a conjugate acid base pair in the following equation?H 3PO 4 + H 2O H 3O +1 + H 2PO 4 -1 What is the pH of a solution whose H +1 concentration is 4.0 X 10 -9? a. 4.0 b. 8.4 c. 3.6 d. 9.0 What is the boiling point of a 2.0 m aqueous solution of sodium chloride?(The boiling point elevation constant for water is 0.512 °C/m.) please answer allarrow_forwardIn the process, H2O + CN- → HCN + OH- , ______ acts a Bronsted-Lowry base. A. H2OB. CN- C. HCND. H3O+E. OHarrow_forward
- Put the following in order from highest to lowest pH if they all have the same molarity: C6H5COOH HClO3 CsOH Ba(OH)2 KCl Li3PO4 Please clearly explain how you got your answer.arrow_forwardThe pH of a 0.92M solution of hypobromous acid (HBRO) is measured to be 4.34. Calculate the acid dissociation constant K, of hypobromous acid. Round your answer to 2 significant digits. K %3D a x10 Canti 2021 McGraw Hill LLC.arrow_forwardIs a solution with pOH = 12.0 a. basic b. acidic c. neutralarrow_forward
- A 1.0-liter solution contains 0.25 M HF and 0.41 M NaF (Ka for HF is 7.2 x 10-4). What is the pH of this solution? O a. 3.36 O b. 10.64 O c. 2.93 O d. 3.14 O e. 0.21arrow_forwardClassifying Salt Solutions as Acidic, Basic, or Neutral Determine whether the solution formed by each salt is acidic, basic, or neutral. 1. SrCl₂ 2. CH³NH₂NO3 3. NH F 4. NaHCO3 K₂ of NH3 = 1.76 x 10-5 K₂ of HF = 3.5 x 10-4 earrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY