
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
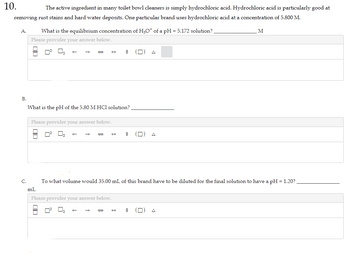
Transcribed Image Text:10.
The active ingredient in many toilet bowl cleaners is simply hydrochloric acid. Hydrochloric acid is particularly good at
removing rust stains and hard water deposits. One particular brand uses hydrochloric acid at a concentration of 5.800 M.
A.
B.
What is the equilibrium concentration of H₂O* of a pH = 5.172 solution?
Please provider your answer below.
What is the pH of the 5.80 M HCl solution?
Please provider your answer below.
☐☐
S
+
00
S
C. To what volume would 35.00 mL of this brand have to be diluted for the final solution to have a pH = 1.20?
mL
Please provider your answer below.
M
$
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following reaction at equilibrium. CH,COOH(aq) = CHyco0 (aq) + H*(aq) When NaCH3CO0 is added to the solution will the value of the equilibrium constant, K, and the pH of the solution increase, decrease, or remain the same? Select one: O A. K, decreases and pH increases O B. Ka remains the same and pH decreases O C. Ka decreases and pH remains the same O D. K, remains the same and pH increases O E. K, increases and pH decreasesarrow_forwardThe Ka for a weak acid is 1.2*10^-8. a. What is the equilibrium concentration of OH- in a 0.0020 M solution of the conjugate base? b. What is the equilibrium concentration of the undissociated conjugate base in a 0.0020 M solution of the conjugate base? c. What is the equilibrium pH of a 0.0020 M solution of the conjugate base?arrow_forwardA sample of water from a stream has [H3O*]=3.1 × 10-9 M. Which statement below accurately describes this water sample? A. The water sample is acidic. B. The water sample is a basic solution. C. The pOH of the water sample is 8.50. D. pH of the water sample is much les than 7.0. E. The pH of the water sample is 7.5.arrow_forward
- b. I'm falling asleep.... The CO₂ concentration in a classroom can increase over the lecture period due to poor ventilation. You decide to measure the pH of pure water in contact with the atmosphere as an indirect way to determine whether you have adequate ventilation. You measure a pH value of 5.3. Assuming equilibrium, i. What is the concentration of CO2 in the classroom? ii. How does the value you calculated compare to the recommended safe level of 800-1000 ppm?arrow_forwardA chemist dissolves 576. mg of pure nitric acid in enough water to make up 150. mL of solution. Calculate the pH of the solution. Be sure your answer has the correct number of significant digits. 0 X Sarrow_forwardA. What is the pH of an aqueous solution with a hydrogen ion concentration of [H*] = 8.1 x 10-5 M? pH B. What is the hydroxide ion concentration, [OH], in an aqueous solution with a hydrogen ion concentration of [H*] = 8.1 x 10-5 M? [OH-] = C. A monoprotic weak acid, HA, dissociates in water according to the reaction HA(aq) = H*(aq) + A-(aq) The equilibrium concentrations of the reactants and products are [HA] = 0.190 M, [H*] = 4.00 x 104 M, and [A-] = 4.00 x 104 M. Calculate the Ką value for the acid HA. Ka = TOOLS x10"arrow_forward
- General Chemistry 4th Edition McQuarrie Rock Gallogly University Science Books presented by Macmillan Learning The K value for acetic acid, CH, COOH(aq), is 1.8 x 10-5. Calculate the pH of a 2.60 M acetic acid solution. pH = %3D Calculate the pH of the resulting solution when 2.50 mL of the 2.60 M acetic acid is diluted to make a 250.0 mL solution. pH = Question Source: MRG - General Chemistry Publish privacy policy terms of use contact us help about us careers N EV prime video P. IIarrow_forwardCalculate the pH of each of these solutions. is the solution acidic, basic, or neutral ?. a. a solution with a OH- concentration of 5.5 x 10-12 M b. a solution with a H3O+ concentration of 3.2 x 10-8 M c. a solution with a pOH of 7.00arrow_forwardUsing the following K, values, calculate the pH values for each of these solutions (using 2 significant figures). [K, for acetic acid= 1.78x105; K, for phosphoric acid = 7.52x103K, for H₂CO3 = 4.3x107] A. A solution of 0.3 M acetic acid has a pH of B. A solution of 3 M acetic acid has a pH of i C. A solution of 100 mM phosphoric acid has a pH of D. A solution of 43 mM H₂CO3 has a pH ofarrow_forward
- 1. A solution of Ba(OH)2 has a [OH-] of 0.40 M. What is the initial molar concentration of the solution? 2. A solution of LiOH has a pH of 13.68. What is the initial concentration of the solution. 3. A solution of Hbr has a [OH-]= 5.0 x 10-12 M. What is the pH of the solution? 4. The [OH-] of CsOH is 0.025 M. What is the pH of the solution? 5. What is the initial concentration of a solution of CsOH with a ph=13.68? 6. A solution of HCl has a pOH=12.08. What is the initial concentration of the solution? 7. The Ka of acetic acid is 1.8x10-5 at 25 degrees C. What is the pH of 0.25 M CH3COOH? 8. The Kb of ammonia is 1.8 x 10-5 at 25 degrees C. What is the pH of 0.25 M NH3?arrow_forwardA. What is the pH of an aqueous solution with a hydrogen ion concentration of [H+]=7.2×10−5[H+]=7.2×10−5 M? B. What is the hydroxide ion concentration, [OH−][OH−], in an aqueous solution with a hydrogen ion concentration of [H+]=7.2×10−5[H+]=7.2×10−5 M? C. A monoprotic weak acid, HAHA, dissociates in water according to the reaction HA(aq)↽−−⇀H+(aq)+A−(aq)HA(aq)↽−−⇀H+(aq)+A−(aq) The equilibrium concentrations of the reactants and products are [HA]=0.120 M[HA]=0.120 M, [H+]=3.00×10−4 M[H+]=3.00×10−4 M, and [A−]=3.00 ×10−4 M[A−]=3.00 ×10−4 M. Calculate the ?aKa value for the acid HA.arrow_forwardChemistry Questionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY