Question
Transcribed Image Text:ississippi College- CHE 124A - Spring21 - SMITH > Activities and Due Dates > HW 9
O Assignment Score:
Lx Give Up?
42.5%
Resources
0%
< Question 17 of 28
n Progress
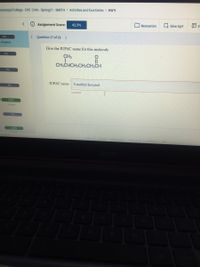
Give the IUPAC name for this molecule.
0%
CH3
CH3CHCH2CH2CH2CH
0%
IUPAC name: 5-methyl-hexanal
0%
Incorrect
100%
Correct
0%
100%
caleeia
pmacy polloy
terme of use contact us help
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Which of the three species is the least stable due to bond order: 0,, 0,, 0 . Hint it may be helpful to draw molecular orbitals for each species although it is not required.arrow_forwardUsing the relative reactivity values shown below, estimate the % yield of each of the two monobrominated product of butane. Relative reactivity values: 3°= 1600, 2°= 82, 1°= 1arrow_forwarda) Explain the dissolution process of a polymeric material of high molar mass into a solvent to from a solution b) what can potentially hinder the process of dissolution of a polymerarrow_forward
- Solutions of ammonia (NH3) and water can be purchased at most grocery stores. What best explains why ammonia can dissolve in water? O Ammonia is a polar covalent compound. O Ammonia is a nonpolar covalent compound. O Ammonia is an ionic compound. O Pure ammonia is a solid at room temperature.arrow_forward50 grams of KCl are dissolved in 200 grams of water at room temperature. Is the resulting solution unsaturated, saturated, or supersaturated. Use the graph belowarrow_forwardArsenic acid is a triprotic acid with the pKa values pKa = 2.240, pK2 = 6.960, and pK 11.500. You wish to prepare 1.000 L of a 0.0100 M arsenate buffer at pH 6.380. To do this, you choose to mix the two salt forms involved in the second ionization, NaH, AsO, and Na, HASO,, in a 1.000 L volumetric flask and add water to the mark. What 4 mass of each salt will you add to the mixture? mass NaH, AsO, = mass Na, HASO, enarrow_forward
- If an electron is removed from each molecule, it is observed that N2+ has a weaker bond than N2, but O2+ has a stronger bond than O2. Explain why electron removal has a different effect on these two molecules.arrow_forwardAll of the following are bases except A. NaOHB. Al(OH)3C. HBrD. Ca(OH)2arrow_forwardA. saturatedB. unsaturatedC. supersaturatedD. You cannot tell by the grapharrow_forward
arrow_back_ios
arrow_forward_ios