
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
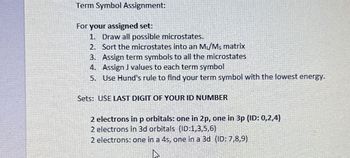
Transcribed Image Text:Term Symbol Assignment:
For your assigned set:
1. Draw all possible microstates.
2. Sort the microstates into an M₁/Ms matrix
3. Assign term symbols to all the microstates
4. Assign J values to each term symbol
5. Use Hund's rule to find your term symbol with the lowest energy.
Sets: USE LAST DIGIT OF YOUR ID NUMBER
2 electrons in p orbitals: one in 2p, one in 3p (ID: 0,2,4)
2 electrons in 3d orbitals (ID:1,3,5,6)
2 electrons: one in a 4s, one in a 3d (ID: 7,8,9)
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- The acidic hydrogen in benzoic acid is IR active at 3573 cm 1. Assuming this hydrogen behaves as a quantum harmonic oscillator, calculate the following (your final answer must be a numerical value). Hints: use the mass of a single hydrogen atom in kilograms and use the frequency, v, in s. 1. ao 2. a 3. az 4. as 5. Normalization constant for wo(x). 6. Normalization constant for w₁(x). 7. Normalization constant for w₂(x). 8. Normalization constant for ws(x).arrow_forwardThe approximate energy of a system is given by (H) =3a4-4a3-36a2+10 where a is a variational parameter. What is the highest energy this system can possibly have in its ground state?arrow_forward1. Using the values from the helium emission spectrum, generate a calibration curve on Microsoft Excel or other graphing software. If you are new to Microsoft Excel, specific instructions (with pictures) are given at the end of this lab and will be discussed in lab. a) In Excel, create a column labeled "scale position." This will serve as your x-values. Create another column labeled "wavelength." This will serve as your y-values. b) Enter the values for your scale position and wavelengths into each column. Do not put unit labels with the numbers. c) Create four separate graphs, one each for the four required types of trendlines ("linear", "logarithmic", "exponential", and "polynomial"). Each graph will have the same data plotted but have a different trendline. See the "Curve Fitting Using an Excel Spreadsheet" section at the end of this write-up for additional information about how to make these graphs. i. ALL graphs must conform to the requirements discussed in lab. 1) graph must take…arrow_forward
- Please show work with explanationarrow_forwardWhich of the following would be classified as a conjugated pi electron system? I II II IV A. II В. IV С. I D. IIarrow_forwardArsenic, with configuration 3p³, has the term symbols 2P, 2D, 4S. Find the possible J values for each of these, and match the appropriate set of J values with each term symbol. 2p I 2D 45 A. 112. B. 312 512 1123 D. 112 312arrow_forward
- Use the equipartition principle to estimate the value of γ = Cpm/CVm for gaseous N2O5. Do this calculation WITH the vibrational contribution to the energy. a. 1.07 b. 1.33 c. 1.4 d. 1.7 e. 1.5arrow_forwardGg.131.arrow_forward13. The infrared absorption spectrum of H2 has a strong band at 4401 cm-1. a. Calculate the force constant of the bond in this molecule. Give your result in units of N m-1. b. Determine the zero-point energy for this molecule. Give your result in units of J. c. At what frequency (in Hz) should the infrared spectrum of D, have a strong band? Report your result in units of cm-1.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY