
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
solve Question 2
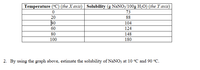
Transcribed Image Text:Temperature (°C) (the X axis) | Solubility (g NaNO3/100g H2O) (the Y axis)
73
20
88
40
104
60
124
80
148
100
180
2. By using the graph above, estimate the solubility of NaNO3 at 10 °C and 90 °C.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- In the McCabe–Thiele method, are the stages stepped off from the top down or the bottom up? In either case, when is it best, during the stepping, to switch from one operating line to the other?Why?arrow_forwardDescribe one key decision that was made either in the design of the task, system, or execution of plant operation that led to the massive gas leak.arrow_forwardHow does acid error & base error affect the pH readings on a pH meter? a. Acid error decreases pH at low pH; base error increases pH at high pH b. Acid error increases pH at low pH; base error decreases pH at high pHarrow_forward
- Q2/A) Reduce the following below using Masson's method: H9 G3 -H3 -H₁ H₂ H7 -H8 G10 G9 G5 G6 G H4 -H5 H6.arrow_forwardA cube of metal is 1.32 cm on an edge. It has a mass of 21.3 g. Calculate the density of this metal cube. Show work for each step including the units in each step.arrow_forwardQUESTION 2 Answer the questions asked using the following property table: Viscosity (N-s/m') Vap 8.020E-06 Prandti No. k (W/m-K) Vap Specific Vol (m'/kg) Vap 206.3 181.7 Watar Properties amp (K) Temp [C | Density (kg/m) AH Vap (k/kg) Cp (k/kg-K) Vap Vap Liq 0.569 1.82E-02 0.574 183E-02 Liq 12.99 Lig Lig 4.217 4.211 Liq Vap Liq 0.001 0.815 1.854 1.750E-03 1855 1.652E-03 8.090E-06 1858 1.422E-03 8.290E-06 1.861 1.225E-03 8.490E-06 1.864 1.080E-03 8.690E-06 9.590E-04 8.890E-06 8.550E-04 9.090E-06 1000.0 0,00485 1000.0 2502 273.15 275 280 285 290 12.22 0.817 0.0055 1000.0 0.00767 1000.0 0.01006 999.0 0.01435 998.0 0.01925 1.85 6.85 0.001 2497 0.582 186E-02 0.590 189E-02 0.598 1.93E-02 0.606 1.95E-02 0.613 196E-02 0.825 0.833 0.841 10.26 2485 2473 2461 0.001 130.4 4,198 881 11.85 0.001 99.4 4.189 7.56 16.85 0.001 69.7 4.184 0.849 0.857 0.865 6.62 2449 2438 4.181 1.868 0.001 0.001 295 21.85 51.94 997.0 0.02556 995.0 0.03362 4.179 4.178 5.83 5.2 1.872 300 305 39.13 29.74 26.85 2426 1.877…arrow_forward
- What is a residue-curve map?arrow_forwardQ2/B) Is the following system under damping? If yes, determine the maximum over shoot, peak time, rise time, and the settling time. X(s) + 2S S2 8S+16 0.1S+1 S+4 Y(s) Sarrow_forwardgrade 12 chemistry: show all work and final answers must have correct units and significant figures. Derive the units of the rate constant k, with respect to an overall 1st order rate law equation. Show all work.arrow_forward
- Q4/ Reduce the following system by using Masson's method: G7 H8 G5 G6 G4 G₁ G2 G3 H4 H5 H6 H₁ H₂ H3 G8 H7arrow_forwardkindly help me with this problem Use free floating decimals in all your calculations and in expressing your final answers.• Solve problem SYSTEMATICALLY and NEATLY thank you! kindly follow this format GIVEN,REQUIRED,SOLUTIONarrow_forwardQ2/A) Reduce the following below using Masson's method: G7 G6 G5 G1 G2 G3 G4 H₁ H₂ H3 H4 H5 H6arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The