
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
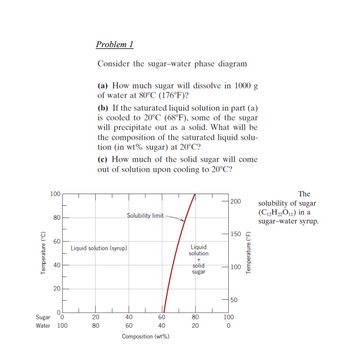
Transcribed Image Text:Temperature (°C)
100
80
60
40
20
0
Sugar
Water 100
0
Problem 1
Consider the sugar-water phase diagram
(a) How much sugar will dissolve in 1000 g
of water at 80°C (176°F)?
(b) If the saturated liquid solution in part (a)
is cooled to 20°C (68°F), some of the sugar
will precipitate out as a solid. What will be
the composition of the saturated liquid solu-
tion (in wt% sugar) at 20°C?
(c) How much of the solid sugar will come
out of solution upon cooling to 20°C?
Solubility limit
Liquid solution (syrup)
20
80
40
60
60
40
Composition (wt%)
Liquid
solution
+
solid
sugar
80
20
||
200
150
100
50
100
0
Temperature (°F)
The
solubility of sugar
(C₁₂H₂2O₁1) in a
sugar-water syrup.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Ethylene is heated at a constant pressure of 5 MPa and 20 degrees Celsius until the temperature reaches 200°C. Using the generalized compressibility diagram, determine the specific volume change in ethylene. FOLLOW THE NEXT STEPS Step 1. Sketch/Paraphrase (Draw the sketch and process diagram if necessary) 2. Theoretical Concepts / Formulas (write down the concepts you are applying and theformulation that will help you solve the problem 3. Information (tables, data in the program, graphs) Assumptions (In the event that they arenecessary) 4.Development Solution (Pay special attention to the units shown and requested)arrow_forwardShown to the right is the solid-liquid phase behavior for mixtures of component A and component B at 1 atm. Use the phase diagram to answer the following questions. a) Specify the melting point of substance B at 1 atm b) What is the maximum composition (mole fraction) of component B that is possible for the mixture to exist in phase S2? At what temperature does this occur? c) 20mol of component A and 180mol of component B are mixed at 350°C and 1 atm. The mixture is cooled at constant pressure to 200°C. i) What phases are present at the final state? ii) What is the composition of each phase at the final state? iii) What is the number of moles of each phase at the final state?arrow_forwardA cylinder initially has a volume of 0.025 m3 that contains 1.5 moles of gas at a temperature of 0°C at a pressure of 1.35 atm. (1) If the pressure is changed to 0.1 atm, but the amount of gas and the temperature are kept constant, what must the new volume be? (2) Starting from the initial cylinder's conditions above, if an additional 1.2 moles of gas are added to the cylinder, in order to keep the volume and pressure constant, what must the new temperature of the gas be? (3) Starting from the initial cylinder's conditions above, if the volume is changed to 0.1 m3 and temperature is increased to 200°C but the amount of gas in the cylinder is unchanged, find the new pressure in the cylinder.arrow_forward
- In an electrolysis of water experiment, 39.75 mL of H2 gas was collected over water at 60.0 °C and an atmospheric pressure of 1.03 atm. The vapour pressure of water at 60.0 °C is 19.92 kPa. What mass of H2 was collected?arrow_forwardUse the Cu-Ag Phase Diagram below to answer the following questions Composition (at% Ag) 20 40 60 80 100 2200 1200 A 2000 Liquidus 1000 Liquid 1800 Solidus C F a +L 1600 * 800 779 C (T) B+L E G 91.2 (Ceg 8.0 71.9 1400 (Cg). 1200 600 Solvus 1000 a +B 800 400 600 200 o 400 100 20 40 60 80 (Cu) Composition (wt% Ag) (Ag) Temperature ("C) Temperature ("F)arrow_forwardHelp me pleasearrow_forward
- 100 atm 1 atm 0.118 atm 114 °C 184 °C 535 °C Temperature (not to scale) a.) Use the above generic phase diagram, clearly identify where you would find the following: Gas, Liquid, Solid, Triple Point, Draw arrows and label to indicate the six transitions (melting, freezing, sublimation, deposition, vaporization and condensation) b.) Based on the above phase diagram, what phase would you be in at a pressure of 50 atm and 300°C? c.) Based on the above phase diagram at what temperature in °C would vaporization occur under normal conditions? d.) If the pressure was 0.050 atm and 425°C, what phase would you be in? e.) If the pressure was 0.118 atm and temperature was 114°C, what phase(s) would you be in? f.) At approximately what temperature would the normal freezing point be in °C? g.) If the pressure was 0.105 atm and starting at a temperature of 32°C to 450°C what phase changes would occur (put in increasing temperature order). Pressure (not to scale)arrow_forward1.00×104 cm³ of 200 °C steam at a pressure of 10.0 atm is cooled until the volume of the liquid water? condenses. What is Part A Give your answer in cm³. Express your answer with the appropriate units.arrow_forwardDetermine the composition and fraction with 50% Ni and 1300 ° Carrow_forward
- According to the following graph, two samples of 1080 steel are cooled from the eutectoid temperature, one at a cooling rate of 250°C/s and the other at a cooling rate of 7.27x10-8 °C/s. Specify the phases obtained and explain their formation from thermodynamic and kinetic perspectives. Also, briefly describe their formation. Draw the microstructure of the phases obtained. Sıcaklık (C) 800 700 600 500 400 300 200 100 0 10 1 T M(başlama) M(% 50) M(% 90) 10 M+O -Otektoid Sıcaklık 10² Zaman (s) % 50 10³ 104 105arrow_forwardAs you know, property diagrams are an important tool used in the study of engineering thermodynamics. In this problem, you will practice using the T-s diagram in conjunction with currently-more-familiar property diagrams. For each part below, sketch T-s, T-v, and P-v diagrams. Clearly indicate the initial and final states, the values of the relevant intensive properties, and the process path on each diagram. Figure 7-11 in your textbook might be helpful in deciding the curvature of some of the process paths. a) Water is taken isobarically from a saturated liquid at 275 kPa to a final state with a quality of 65%. b) Water, initially at 2 MPa and 500degrees C, in a rigid tank eventually comes to thermal equilibrium with the surroundings at 100degrees Cc) Refrigerant-134a is compressed isobarically from a saturated vapor with h=256.22 kJ/kg to a final temperature of 0degrees Carrow_forwardGive me right solution with clear calculationsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY