
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
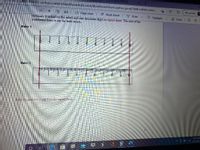
What is the margin of error[ in each ruler
Transcribed Image Text:eslions | bartieby
fleet01-xythos.content.blackboardcdn.com/blackboard.learn.xythos.prod/584b1d8497c84/1... to
由
Not syncing
E D Page view A Read aloud V Draw v
I Highlight
O Erase
Icertainty (marked on the nuler) and one uncertain digit on report sheet. The unit of the
numbered lines is cm for both rulers.
on the teport sheet.
11:27 AM
2/14/202
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give detailed Solution...don't give Handwritten answer..don't use Ai for answering thisarrow_forwardGive correct detailed Solution..show work..don't give Handwritten answer..don't use Ai for answering thisarrow_forwardIn 38.7 mL of water, a gold nugget weighing 523 g is inserted. The volume of the water increases to i.e. 70 mL. I'm curious in the gold's density.arrow_forward
- Two beakers contain clear colorless liquids, when the contents of the beakers are mixed,a white solid is formed (a)is this an example of a chemical or or a physical change(b) what would be the most convenient way to separate the newly formed white solid form the mixture-filtration, distillation, chromatography. elaborate your answerarrow_forwardA dose of aspirin of 5.0 mg per kilogram of body weight has been prescribed to reduce the fever of an infant weighing 8.5 pounds. The number of milligrams of aspirin that should be administered is (note 2.201 lb = 1 kg)arrow_forwardGiven that 1 inch = 2.54 cm, 1.00 cm³ is equal toarrow_forward
- On average, a 1.3 gram tablet has 500. milligrams of calcium carbonate. What is the mass/mass percent of calcium carbonate in the tablet?arrow_forwardHi, I'm learning about Density in chemistry , and I'm confused about the exemplos that the professor give me about significant figure, because we told us that 0 is only significant if there is a number bigger then 1 in front . But on the example he give us, the first one he left the 0 but on the second he did not !!arrow_forwardAlie measures out a sample of naphthalene using a graduated cylinder. She reads the measurement as 9.84 mL. The actual volume is 10.29 mL. What is her percent error?arrow_forward
- Round 0.00971659 to three significant figures.arrow_forwardI need the answer as soon as possiblearrow_forwarda prescription requires 7.55mg per kg of body weight. Convert this quantity to the number of grams required per pound of body weight and determine the coreect dose in grams for a 175 lb patient. Iseem to be having a difficult time with the conversions....arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY