
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
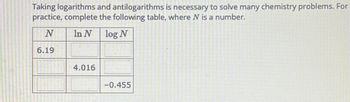
Transcribed Image Text:Taking logarithms and antilogarithms is necessary to solve many chemistry problems. For
practice, complete the following table, where N is a number.
N
In N
log N
6.19
4.016
-0.455
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 2arrow_forward15 16 17 18 19 20 22 23 24 26 27 A chemist prepares a solution of aluminum chloride (AICI,) by measuring out 23. g of aluminum chloride into a 250. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's aluminum chloride solution. Round your answer to 2 significant digits. mol/L OnD Continue Submit Assignment O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use 1 Privacy Center Accessibility S0 F5 F6 F8 %23 $ % & 4 5 6 7 8 25 21 %#3 2.arrow_forwardA chemist measures the amount of lodine solid produced during an experiment. He finds that 3.02 g of lodine solid is produced. Calculate the number of moles of iodine solid produced. Be sure your answer has the correct number of significant digits. mot X 5 ?arrow_forward
- Part 1 of 2 Determine the pH of a 0.30 M NH3 solution. Be sure your answer has the correct number of significant digits. -5 Note: K for NH₂ is 1.78 × 10¯ pH = x10 X Sarrow_forwardTry Again Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. A chemist prepares a solution of iron (II) chloride (FeCl₂) by measuring out 1.0 g of FeCl₂ into a 250. mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of C1 anions in the chemist's solution. Be sure your answer is rounded to 2 significant digits. 1.5 × 10 -2 mol L x10 X Śarrow_forwardPART III: QUESTIONS Question 1: a ‘law’ in science is a general statement that is the conclusion of multiple observations. Formulate a law that describes what happens when a metal chloride solution is mixed with a silver chloride solution. Question 2: Formulate a law that describes what happens when a metal chloride solution is mixed with a sodium phosphate solution. Question 3: If an aqueous solution of titanium chloride, which is clear and purple, is mixed with a silver nitrate solution, predict what precipitate will form. Question 4: If an aqueous solution of titanium chloride, which is clear and purple, is mixed with a sodium phosphate solution, predict what precipitate will form.arrow_forward
- CHEMICAL REACTIONS Dilution A chemist must dilute 40.8 mL of 4.55 M aqueous zinc nitrate water to the solution until it reaches a certain final volume. Calculate this final volume, in milliliters. Be sure your answer has the correct number of significant digits. X S 0/3 (Zn(NO3)₂) solution until the concentration falls to 4.00 M. He'll do this by adding distilledarrow_forward27 A chemist prepares a solution of sodium chloride (NaCl) by measuring out 0.50 g of NaCl into a 250. mL volumetric flask and filling to the mark with distilled water. Calculate the molarity of Cl anions in the chemist's solution. do Be sure your answer is rounded to 2 significant digits. 18 Ar mol x10 L Submit Assignment Continue 2022 McGraw Hill LLC. AIl Rights Reserved. Terms of Use Privacy Center Accessibility Show All IMG-6095.jpg IMG-6096.jpg IMG-6097.jpg IMG-6098.jpg IMG-6099.jpg MacBook Air DD DII F12 F11 F10 F9 80 F7 F8 F6 F5 F4 esc F2 F3 F1 & % @ # 7 8 3 4 5 6 1 2 { P E R Y Q W %24arrow_forwardCan someone please help compete the last tablearrow_forward
- A chemist measures the amount of fluorine gas produced during an experiment. He finds that 3.1 g of fluorine gas is produced. Calculate the number of moles of fluorine gas produced. Round your answer to 2 significant digits. Ú mol 0 x10 Xarrow_forwardIs distillation a good method for separating a mixture of water and hexane? Why or why not?arrow_forwardBased on this. What mass of water was driven off by heating? (Grams)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY