
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
solve using bayes' rule
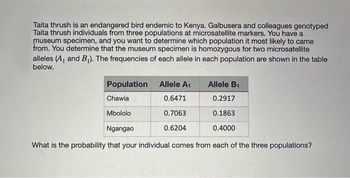
Transcribed Image Text:Taita thrush is an endangered bird endemic to Kenya. Galbusera and colleagues genotyped
Taita thrush individuals from three populations at microsatellite markers. You have a
museum specimen, and you want to determine which population it most likely to came
from. You determine that the museum specimen is homozygous for two microsatellite
alleles (A₁ and B₁). The frequencies of each allele in each population are shown in the table
below.
Population
Chawia
Mbololo
Allele A₁
0.6471
0.7063
0.6204
Allele B₁
0.2917
0.1863
0.4000
Ngangao
What is the probability that your individual comes from each of the three populations?
Expert Solution
arrow_forward
Step 1: The probability of being homozygous for allele B ( BB) is the square of the frequency of allele B.

Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Similar questions
- The following table shows experimental results of the glucose transport rate, mM/sec, following facilitated diffusion by glucose carrier proteins. (Recall: the starting conc. L represents glucose added to one side of the membrane; distilled water, omM of glucose was added to the other side of the membrane). The rate of glucose transport was 0.0031 mm/sec with 8mM of glucose (run number 4, highlighted); the rate decreased to 0.0017 mM/sec with 10mM of glucose (run 5, highlighted). Why was the rate of glucose transport slower when the concentration gradient was increased? Experiment Results Run Number Solute 1 1 2 2 3 33 4 4 5 6 6 Na Ch Glucose Na Ch Glucose Na Ch Glucose Nat Ch Glucose Na Ch Glucose Nat Cl Glucose Start Conc. L Start Conc. R (MM) (mM) 0.00 0.00 2.00 0.00 0.00 0.00 8.00 0.00 0.00 0.00 2.00 0.00 0.00 0.00 8.00 0.00 0.00 0.00 10.00 0.00 2.00 0.00 2.00 0.00 Carriers 500 500 500 500 700 700 700 700 100 100 700 700 Rate (mm/sec) 0.0000 0.0008 0.0000 0.0023 0.0000 0.0010…arrow_forwardif the assumption for hardy-weinberg are always being violated, then how can these equations still be useful? Quizletarrow_forwardSolve correctly please,with explanationarrow_forward
- The folding and unfolding rate constants for a myoglobin mutant have been determined. The unfolding rate constant ke-u = 3.62 x 10-55 and the folding rate constant ku-p = 255 s1, where Fis the folded protein and U is the unfolded (denatured) protein. For wild-type myoglobin, AG;u = +37.4 kJ/mol. Which myoglobin is more thermodynamically stable, the mutant or the wild-type?arrow_forwardBriefly explain why a "Folding Funnel" is used to represent the kinetics of folding (limit 5-6 sentences)?arrow_forwardA research group discovers a new version of happyase, which they call happyase*, that catalyzes the chemical reaction: HAPPY-SAD The researchers begin to characterize the enzyme. The researchers determined the kcat of the enzyme to be 452 s-1. In a separate experiment with [E;) at 1.5 nM and [HAPPY) at 38 µM, the researchers find that Vo is equal to 320 nM s-1. What is the measured Km of happyase* for its substrate HAPPY? Make sure to include units in the answer.arrow_forward
- Identify the incorrect statement regarding the polypeptide, Myoglobin. Select one: a. The interaction between Myoglobin and O2 is homotopic in nature as indicated by a Hill coefficient n = : 1 b. Myoglobin is found predominantly in muscle tissue because it facilitates oxygen diffusion. c. The majority of Myoglobin's secondary structure is composed of alpha-helices d. Myoglobin has a higher affinity for oxygen than Hemoglobin e. Myoglobin serves as an oxygen storage protein as suggested by its hyperbolic binding of 02arrow_forwardIntestinal epithelial cells pump glucose into the cell against its concentration gradient using the Na*-glucose symporter. Recall that the Na+ concentration is significantly higher outside the cell than inside the cell. The symporter couples the "downhill" transport of two Na+ ions into the cell to the "uphill" transport of glucose into the cell. If the Na+ concentration outside the cell ([Na* lout) is 147 mM and that inside the cell ([Na+]in) is 17.0 mM, and the cell potential is -54.0 mV (inside negative), calculate the maximum energy available for pumping a mole of glucose into the cell. Assume the temperature is 37 °C. AG gluc kJ mol What is the maximum ratio of [glucose]in to [glucose] out that could theoretically be produced if the energy coupling were 100% efficient? 1.13 2.3 × 10-4 8.36 4300arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education