
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Table of
Transcribed Image Text:T
h
h
(K)
(°C)
(kJ/kmol)
(kJ/kmol)
(kJ/kg)
(kJ/kg)
200
-73.15
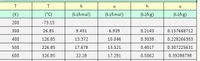
Transcribed Image Text:h
h
(K)
(°C)
(kJ/kmol)
(kJ/kmol)
(kJ/kg)
(kJ/kg)
200
-73.15
300
26.85
9.431
6.939
0.2143
0.157668712
400
126.85
13.372
10.046
0.3038
0.228266303
500
226.85
17.678
13.521
0.4017
0.307225631
600
326.85
22.28
17.291
0.5062
0.39288798
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- 1) For work done in an isobaric process, there is 2 equations equal to each other to find the answer (seen from the photo) but as I used those 2 equations I got 2 different values. Can someone help? 2) There is a phase change from solid to liquid which is isothermal. However, is it also an isobaric process? If not, what is the pressure at the solid state?arrow_forwardWhat is a minimum-boiling azeotrope? What is a maximum-boiling azeotrope? Which type is by far the most common?arrow_forwardComplete the table below: Dipole Moment (debye) 9-Fluorenone 3.06 Benzoic Acid 4.80 a) What does a dipole moment value represents for a compound? b) How the dipole moments for 9-Fluorenone and Benzoic Acid compare? What does it mean? Think in the Partition coefficient labs that you already performed.arrow_forward
- A 0.103-m³ tank contains 8.6x1026 molecules of N₂ at 17 MPa. Compute the (a) specific volume. Also, determine the temperature in the tank using (b) the Van der Waals equation, (c) Beattie- Bridgeman equation and (d) Benedict-Webb-Rubin equation of state. Compare each temperature you obtained with the actual value (which is 182.8 K) by computing the % error.arrow_forwardSea water at 20ºC and 1 bar. If the salt mole fraction in the water is 0.01, what is the entropy of H2O in the sea water? Note: the question can be analyzed by the ideal solution, and it is as follows: Si=niSmi0-niRlnxarrow_forwardPlease explain it well, thank you so much.arrow_forward
- How did you assume the coefficient of discharge Cd? Did you get it from a table? Thxarrow_forwardShort Answer: When you add salt to water, the boiling point is elevated. Briefly connect this to ideal solutions vs non-ideal solutions and mention how inter-molecular forces are involved. If two components were added together and the resulting mixture approximated an ideal solution, what do you expect to be true about the molecular nature of the two components, do you expect them to be similar or dissimilar, why?arrow_forwardDescribe the significance of the Gibbs theorem for ideal gases.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The