
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
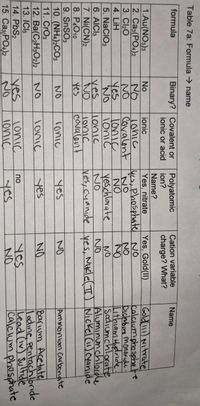
Transcribed Image Text:Table 7a: Formula → name
formula
Binary? Covalent or
Polyatomic
ion?
Cation variable
Name
ionic or acid
charge? What?
Name?
1.Au(NO3)3
2. Сaз(РO,)2
3. Cl20
4. LiH
No
ionic
Yes, Gold(II)
Gold 111) Nitrate
calcıumphosphate
Dichbire monor ide.
Lithiam Hudride.
Sadiumchlorate
Aluminunchlorde
yes, Nklel TT) NIckel (u) Cyanide
Yes, nitrate
NO
NO
yes
1onic
Covalent
loniç
Tonic
onic
lonic
Covalent
yıs,phosphate NO
NO
NO
yeschlorate
NO
NO
5. NaCIO3
6. AICI3
7. Ni(CN)2
8. P4O10
9. SnSO3
10. (NH4)2CO3
11. CrO3
12. Ba(C2H3O2)2
13. ICI5
NO
ves
NO
yes
Nes,cuanide
NO
lonic
Yes
NO
Ammoniom Carbonate
NO
Barium Acetate
lodıne pentachloride
Lead iv) Sultide
|calcium phosphate
NO
lonic
yes
14. PbS2
15. СазРО:)2
yes
lonic
Jonic
yes
NO
no
yes
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using the chart please fill in the names and chemical formula for the 3rd Line (NH41+) line the whole column is 1 question thank you!!!!arrow_forward= O ATOMS, IONS AND MOLECULES Naming ionic compounds with common polyatomic ions Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: cation 2+ Zn 4+ Pb 2+ Mg anion co CIO 3 So Some ionic compounds empirical formula 0 0 name of compound 0 X Sarrow_forward2. Write the formula from the following compound names or the name from the following formula. a) sodium cyanide b) magnesium acetate c) (NH4)2HPO4 d) KHSarrow_forward
- 1. Fill in the correct subscript in the formula for the ionic compound which contains Zn 2+ cation and (CO3) 2- anion. Zn______ (CO3)_______ 2.Name the ionic compound which contains Fe 3+ cation and (PO3) 3- anion. Name:_________________________ 3. Fill in the subscripts in the formula for the following compound Cupric acetate Cu_____ (C______ H______ O_____ )_______ .arrow_forward3. Fill in the empty boxes with the correct information. Cation Ca2+ NH4* Anion C1- COR CIO3 Formula K₂S MgS NA Name Mag Culfide Lithium Carbonate C hllak the periodic table below. Four ofarrow_forwardb Answered: Question 52 of 65 V X 101 Chem101 G chemical formula for the bisulfa X C Question 46 Of 46 Submit Wha X + app.101edu.co M Apps G M Gmail YouTube Маps a AMAZON Translate Gflights USCIS b BATERBLY C CHEGG > KATAPULK CUBA SUPERMARKET23 Reading List Question 46 of 65 Submit Write the chemical formula for the bisulfate ion 4- 2+ 3+ 4+ 1 4 6. 7 8. 9. O3 (s) (1) (g) (aq) Na H. S Ph Si Reset x H,O Delete + LO 2. 4. 3. 2. 2.arrow_forward
- O ATOMS, IONS AND MOLECULES Naming ionic compounds with common polyatomic ions cation Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: K 2+ Zn 2+ Pb anion MnO 4 C103 CH,CO, Some ionic compounds empirical formulat Enter your payment details | bartle name of compound 0/3 Xarrow_forwardName: a. Choose the correct name and write the correct formula for the ionic compound formed when the Fe²+ cation combines with the OH anion. Formula: Formula: b. Choose the correct name and write the correct formula for the ionic compound formed when the A13+ cation combines with the C1O4 anion. Name: Name: Formula: [Review Topics] Submit Answer * c. Choose the correct name and write the correct formula for the ionic compound formed when the Na cation combines with the 02- anion. Retry Entire Group [References] 8 more group attempts remainingarrow_forwardWhat is the formula for the compound formed from Na1+ out the formula in a line, for example Na2CO3 (no subscripts, formulas are case and 02- ions? Please write sensitive!).arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY