
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
"Using the table give Calculate the temperature change for both the water and unknown objects. Show your work for at least one temperature change calculation. Record your values in Table 1."
PLEASE HELP ME WITH THIS TABLE. i really need help and my teacher is not emailing me back! please help me!!
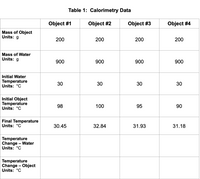
Transcribed Image Text:**Table 1: Calorimetry Data**
| | Object #1 | Object #2 | Object #3 | Object #4 |
|-------------------------|-----------|-----------|-----------|-----------|
| **Mass of Object** | | | | |
| Units: g | 200 | 200 | 200 | 200 |
| **Mass of Water** | | | | |
| Units: g | 900 | 900 | 900 | 900 |
| **Initial Water Temperature** | | | | |
| Units: °C | 30 | 30 | 30 | 30 |
| **Initial Object Temperature** | | | | |
| Units: °C | 98 | 100 | 95 | 90 |
| **Final Temperature** | | | | |
| Units: °C | 30.45 | 32.84 | 31.93 | 31.18 |
| **Temperature Change – Water** | | | | |
| Units: °C | | | | |
| **Temperature Change – Object** | | | | |
| Units: °C | | | | |
**Description:**
- This table presents data collected from a calorimetry experiment involving four different objects.
- Each object has a mass of 200 grams, and each was placed in 900 grams of water.
- The initial and final temperatures of both the water and the objects are recorded in degrees Celsius.
- For the water, the initial temperature is consistently 30°C for all objects.
- Initial temperatures of the objects vary, with Object #1 starting at 98°C, Object #2 at 100°C, Object #3 at 95°C, and Object #4 at 90°C.
- Final temperatures after the experiment are slightly above the initial water temperature for each object, indicating heat transfer.
- The table has sections for temperature changes for both water and objects, but those values have not been filled in.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Use Newton’s Law of Cooling, T = C + (T0 - C)ekt, to solve, A bottle of juice initially has a temperature of 70°F. It is left to cool in a refrigerator that has a temperature of 45°F. After 10 minutes, the temperature of the juice is 55°F. Solve, a. Use Newton’s Law of Cooling to find a model for the temperature of the juice, T, after t minutes. b. What is the temperature of the juice after 15 minutes? c. When will the temperature of the juice be 50°F?arrow_forwardPlease give answer in Handwritten format. This is important question,If you use Chatgpt i will dislike.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON