Question
Transcribed Image Text:The Einstein model for a solid assumes the system consists of 3N independent simple harmonic oscillators with frequencies &. Within these assumptions, the heat capacity at constant volume as:
Cv=3Nk() (-1)²
²
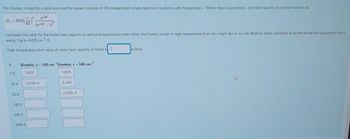
Complete the table for the molar heat capacity at various temperatures under either the Einstein model or high-temperature limit. You might like to use the Wolfram Alpha calculator to do the numerical calculations more
easily. Use k-0.695 cm /K.
High temperature limit value of molar heat capacity of metal is
T
1 K
10 K
50 K
-1
Einstein, = 100 cm Einstein, : = 500 cm
1.4021
3.8991
100 K
500 K
2.434E-4
1000 K
6.1499
2434E-4
kJ/mol.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- A particle has a rest mass of 6.35 × 10¯ kg and a momentum of 5.39 × 10-¹8 kg.m/s. Determine the total relativistic energy E of the particle. 1.67 ×10-⁹ E = Incorrect Find the ratio of the particle's relativistic kinetic energy K to its rest energy Erest. K 1.93 Erest || Incorrectarrow_forward(89) X 0 馄.all 89% 12:40 PM Homework 1 PHYs 2100-09-LAB, SPRING 2019 NAME CIN Question 1. A bird flies a distance of d- 150+3 m during a time 20.0+1.0 s The average speed of the bird is u-d/t = 7.5 m/s. what is the uncertainty of u? Question 2. Suppose that we're able to eliminate the uncertainty in our time mea- surement so that t = 20.0 ±0.0 s. What is the new uncertainty of u?arrow_forwarda) If the mass lost in a nuclear fission reaction is 15 grams, how much energy is released? b) An proton has a mass of 1.6 x 10-27 kg. How much energy would be released if the mass of one proton was lost in a nuclear fission reaction?arrow_forward
- Helpful information: (1) An alpha particle is a helium nucleus, (2) e = 1.6 × 10-¹⁹ C, (3) k = 9.0 × 10⁹ Nm² C-2, (4) 1nm = 1 × 10-⁹ m 1-An alpha particle lies on the x-axis, a distance of 1.0 nanometer from a proton (in this set-up, the alpha particle is at the origin while the proton is in the positive direction). Which of the following choices below represents the magnitude of the electric force on the alpha particle? (a) 2.3 × 10-10 N (b) 4.6 × 10-¹0 N (c) 2.3 × 10-19 N (d) 4.6 × 10-19 N eplacing the voltage sou the following inst choices below time != 1.00 37 instantaneous currentarrow_forwardThe energy of a moving object is given by the equation of E= 12??212mv2 . What is the constant? What type of variation is this? If the energy is 16 joules and the mass is 1 kg (kilogram), what is the velocity?arrow_forward% Difference between largest and smallest velocities = 1A-B1 AAB)X100%= .0293-.4137 x 100%= C0293+o4137arrow_forward
- Bonus Problem 2- We have measured the radius of Earth R= 6400 km. A cosmic particle which is moving with v = 0.95 c (where c is the speed of light) toward the center of Earth. a) What is the diameter of Earth from point of view of the particle! b) How long will it take for the particle to pass through the Earth?arrow_forwarddp" qF" u' (v summation implied), dr Verify that the relativistic force law where E, E, cB. -cB, E. E, %3D Е, -сВ. Е. сВ, -сВ, cBx yields the relativistic Lorentz force equation d(ymv) =q(E+v×B) q(È +vx. dt when evaluated on the space components a = 1,2,3.arrow_forwardA star is 12.2 ly (light-years) from Earth. HINT (a) At what constant speed (in m/s) must a spacecraft travel on its journey to the star so that the Earth–star distance measured by an astronaut onboard the spacecraft is 4.36 ly? m/s (b) What is the journey's travel time in years as measured by a person on Earth? NO SCIENTIFIC NOTATION ANSWERS THANK YOUarrow_forward
- I have asked kindly for answers to be in a manner that I understand. i do not understand Physics. Please explain to me what this is supposed to be in terms that I understand. Thank You. Pi=Pf0=4×10-3×v+6.82×-0.254×10-3×v=6.82×0.25v=6.82×0.254×10-3v=426.25 m/sarrow_forwarddetails explanation, please!arrow_forward
arrow_back_ios
arrow_forward_ios