
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Suppose you have a gas that obeys the following modified van der Waals equation:
P(V-nb)=nRT
a) In 1–2 sentences, provide a physical interpretation for this equation.
HINT: Compare this with the actual van der Waals equation. What does the missing term represent?
b) For a gas obeying this modified van der Waals equation, derive an expression for the work done by a reversible and isothermal change in volume.
c) For this modified van der Waals equation (with n and b as constants), find the following two partial derivatives for i and ii
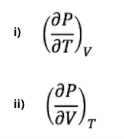
Transcribed Image Text:i)
ƏT.
ii)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider 1.2 moles of a diatomic gas that expands adiabatically from an initial pressure of 5.0 x 10^5 Pa and a temperature of 400 K to a final pressure of 1.0 x 10^5 Pa. a) Find the final temperature. b) Find the final volume. c) Find the work done by the gas. d) Find the change in internal energy of the gas.arrow_forwardHydrogen behaves as an ideal gas at temperatures greater than 200 K and at pressures less than 50 atm. Suppose 6.00 mol hydrogen is initially contained in a 100-L vessel at a pressure of 2.00 atm. The average molar heat capacity of hydrogen at constant pressure, c, is 29.3 J K-' mol-1 in the temperature range of this problem. The gas is cooled reversibly at constant pressure from its initial state to a volume of 50.0 L. Calculate the following quantities for this process. (a) Temperature of the gas in the final state, T2 (b) Work done on the gas, w, in joules (c) Internal energy change of the gas, AU, in joules (d) Heat absorbed by the gas, q, in joulesarrow_forwardConsider the free, isothermal (constant T) expansion of an ideal gas. "Free" means that the external force is zero, perhaps because a stopcock has been opened and the gas is allowed to expand into a vacuum. Calculate AU for this irreversible process. Show that q sion is also adiabatic (q = 0) for an ideal gas. This is analogous to a classic experiment first performed by Joule. 0, so that the expan-arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY