
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
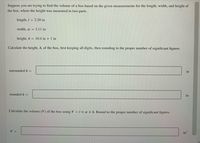
Transcribed Image Text:Suppose you are trying to find the volume of a box based on the given measurements for the length, width, and height of
the box, where the height was measured in two parts.
length, I = 2.20 in
width, w = 3.11 in
height, h = 10.4 in + 1 in
Calculate the height, h, of the box, first keeping all digits, then rounding to the proper number of significant figures.
unrounded h =
in
rounded h =
in
Calculate the volume (V) of the box using V =Ixw x h. Round to the proper number of significant figures.
V =
in
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An iceberg has a volume of 8365 ft^3 What is the mass in kilograms of the iceberg? The density of ice in the iceberg is 0.92 g/cm^3. Express your answer using two significant figures.arrow_forwardhow do i set up the matharrow_forwardA student was given an unknown metal. The student determined that the mass of the metal was 30.2 g. The student placed the metal in a graduated cylinder filled with 20.0 mL of water. The metal increased the volume of water to 22.9 mL. Calculate the density of the metal and determine the identity of the metal using the table below.arrow_forward
- Suppose you are trying to find the volume of a box based on the given measurements for the length, width, and height of the box, where the height was measured in two parts. length, l = 2.20 in width, w = 3.71 in height, h = 10.4 in + 1 in Calculate the height, ℎ, of the box, first keeping all digits, then rounding to the proper number of significant figures. unrounded h = ____in rounded h =____ in Calculate the volume (V) of the box using V =l x w x h. Round to the proper number of significant figures. v=_____in3arrow_forwardplease see attached imagearrow_forwardThe quadratic formula is used to solve for x in equations taking the form of a quadratic equation, ax? + bx + c = 0. -b ± b2 – 4ac quadratic formula: x = 2a Solve for x in the following expression using the quadratic formula. 2x2 + 21x – 7.1 = 0 Use at least three significant figures in each answer. x = and x =arrow_forward
- How many significant figures should your result contain? What is the calculated density? Express your answer to the correct number of significant figures.arrow_forwardIn 1999, scientists discovered a new class of black holes with masses 100 to 10,000 times the mass of our sun, but occupying less space than our moon. Suppose that one of these black holes has a mass of 8×103 suns and a radius equal to one-half the radius of our moon. What is its density in grams per cubic centimeter? The mass of the sun is 2.0×1030kg and the radius of the moon is 2.16×103mi. (Volume of a sphere =43πr3.)arrow_forwardIn 1999, scientists discovered a new class of black holes with masses 100 to 10,000 times the mass of our sun, but occupying less space than our moon. Suppose that one of these black holes has a mass of 1×10^3 suns and a radius equal to one-half the radius of our moon. What is its density in grams per cubic centimeter? The mass of the sun is 2.0×10^30 kg and the radius of the moon is 2.16×10^3 mi (Volume of a sphere =4/3πr^3)arrow_forward
- Perform the calculations below and give the results with correct significant figures. 31.816 g + 4.23 g + 0.097 g = 3.48 m × 5.916 m × 19.27 m = (0.0451 × 2.007 × 104) − (28.16 ÷ 0.874) = g m3arrow_forwardI don't understand the wording. The least significant digit means the last digit? 7.700 X 2.1 = 16.17 would the uncertainty be 0.01 ? 2.132 + 17 + 4.04 = 23.172 would the uncertainty be 0.001 ?arrow_forwardA chemistry student needs 35.0 mL of dimethyl sulfoxide for an experiment. By consulting the CRC Handbook of Chemistry and Physics, the student discovers that the density of dimethyl sulfoxide is 1.10 g·cm -3 Calculate the mass of dimethyl sulfoxide the student should weigh out. Be sure your answer has the correct number of significant digits. ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY