
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
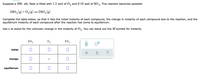
Transcribed Image Text:Suppose a 500. mL flask is filled with 1.3 mol of O, and 0.10 mol of SO3. This reaction becomes possible:
2s0,(g) +0,(g) – 2s0;(g)
Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the
equilibrium molarity of each compound after the reaction has come to equilibrium.
Use x to stand for the unknown change in the molarity of 0,. You can leave out the M symbol for molarity.
2.
so,
02
so,
initial
change
equilibrium
Expert Solution
arrow_forward
Step 1
GIVEN:
Volume of the Flask = 500 mL= 0.5 L
Moles of O2=1.3 mol
Moles of SO3 =0.10 mol
Given reaction is:
2SO2(g) + O2 <------> 2SO3(g)
arrow_forward
Step 2
Concentration = Moles/ Volume (L)
Concnetration of O2 = 1.3 mol / 0.5 L
= 2.6 M
Concentration of SO3= 0.10 mol/0.5 L
= 0.2 M
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A solution contains 20.0% (by mass) of ammonium sulfate, (NH4),SO4, and has density of 1.12 g/mL. (a) What is the mass (in grams) of a 250.-mL sample of this solution? (b) How many grams of (NH4)2SO4 are present in a 250.-mL sample of this solution? (c) Calculate: (i) the molar concentration, and (ii) the molality of this solution.arrow_forwardIf 53.0 g of KClO3 was transferred into 125.0 g of water at 30.0 celcius. how many grams of KClO3 will percipitate? g solute at 30.0 celcius is 10 gramsarrow_forward4arrow_forward
- 5. Sodium acetate can be formed from the metathesis/double replacement reaction of sodium bicarbonate and acetic acid. (See the Supplemental Information concerning metathesis reactions.) Determine the percent yield when 0.537 g of sodium acetate is obtained from mixing 15.0 g of NaHCO3 with 125 mL of 0.15 M acetic acid.arrow_forwardSolubility Curves of Selected Compounds 150 140 130 120 110 100 NaNO, 90 80 70 60 NH C KCI Nac 50 40 30 20 KCIÓ 10 Cez(SO4)3 10 20 30 40 50 60 70 90 100 Temperature (C) The compound whose solubility increases the fastest with an increase in temperature is: OKI O NANO3 O KNO3 O NH, O NH&CI O KCI O NaCI O KCIO, O Ce:(SOala DELL Grams of solute per 100 g H,O NH FONXarrow_forwardSuppose a 500. mL flask is filled with 0.80 mol of CO, 0.60 mol of CO, and 0.90 mol of H,. This reaction becomes possible: Co(g) +H,0(g) =CO,(g) +H,(g) [+(8)' Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of CO. You can leave out the M symbol for molarity. CO H,0 CO, H, initial ? change equilibriumarrow_forward
- 13.arrow_forwardSuppose a 250. mL flask is filled with 0.70 mol of H2 and 1.1 mol of 12. The following reaction becomes possible: H2(g) +I2(g) — 2HI(g) =0 The equilibrium constant K for this reaction is 8.37 at the temperature of the flask. Calculate the equilibrium molarity of 12. Round your answer to two decimal places. Омarrow_forwardFor an experiment you are measuring the decomposition rate of organic matter, You have a large amount of CH402 in a container filled with 3.024 Lof water. You Created a saline environment by adding 2.4 g of sodium chloride, 3.6 g of calcium chloride, and 2.8 g of magnesium chloride before diluting. iron (1) oxide is available for the microbes to oxidize the matter into carbon dioxide by forming iron (II) aqueous ions. At the beginning of the experiment, you measure the pH using a probe to be 7.13. After 73 hours you measure the pH again and obtain 7.54. Assuming that the pH change is solely due to the oxidation of organic matter, what is the rate of organic matter decomposition in nanograms per hour?arrow_forward
- Suppose a 250. mL flask is filled with 1.3 mol of CO, 1.0 mol of CO, and 1.8 mol of H,. This reaction becomes possible: 2 CO (g) +H,0(g) – CO,(g)+H,(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of CO. You can leave out the M symbol for molarity. CO H20 co2 H2 initial ? change equilibriumarrow_forwardSuppose a 250. mL flask is filled with 1.5 mol of CO, 0.70 mol of H,0 and 1.8 mol of H,. This reaction becomes possible: co(g) +H,0(g) =CO,(g)+H,(g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of H,. You can leave out the M symbol for molarity. H,0 co, н, co initial change х equilibriumarrow_forwardSuppose a 500. mL flask is filled with 0.60 mol of H,S, 0.10 mol of CS, and 1.5 mol of H,. This reaction becomes possible: CH (g) + 2H,S (g) =CS,(g)+4H, (g) Complete the table below, so that it lists the initial molarity of each compound, the change in molarity of each compound due to the reaction, and the equilibrium molarity of each compound after the reaction has come to equilibrium. Use x to stand for the unknown change in the molarity of CH4. You can leave out the M symbol for molarity. CHA H,S cs, H2 initial change equilibriumarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY