
Introductory Chemistry: A Foundation
9th Edition
ISBN: 9781337399425
Author: Steven S. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
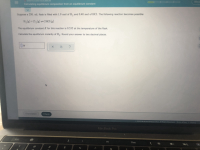
Transcribed Image Text:Suppose a 250. mL flask is filled with 1.9 mol of H, and 0.40 mol of HCl. The following reaction becomes possible:
H,(g) + Cl,(g) -- 2HCI (2)
The equilibrium constant K for this reaction is 0.535 at the temperature of the flask.
Calculate the equilibrium molarity of H,. Round your answer to two decimal places.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In Section 17.3 of your text, it is mentioned that equilibrium is reached in a closed system. What is meant by the term “closed system,” and why is it necessary for a system to reach equilibrium? Explain why equilibrium is not reached in an open system.arrow_forward5.19. Assume that a reaction exists such that equilibrium occurs when the partial pressures of the reactants and products are all . If the volume of the system were doubled, all of the partial pressures would be . Would the system still be at equilibrium? Why or Why not?arrow_forward12.85 In the figure, orange fish are placed in one aquarium and green fish in an adjoining aquarium. The two tanks are separated by a removable partition that is initially closed. Describe what happens in the first few minutes after the partition is opened. WTiat would you expect to see several hours later? How is this system analogous to dynamic chemical equilibrium?arrow_forward
- In Section 13.1 of your text, it is mentioned that equilibrium is reached in a closed system. What is meant by the term closed system. and why is it necessary to have a closed system in order for a system to reach equilibrium? Explain why equilibrium is not reached in an open system.arrow_forwardDescribe a nonchemical system that is in equilibrium, and explain how the principles of equilibrium apply to the system.arrow_forward5.2. What is the difference between a static equilibrium and a dynamic equilibrium? Give examples difference from the examples in the text. What is similar for the two types of equilibria?arrow_forward
- . Many sugars undergo a process called mutarotation, in which the sugar molecules interconvert between two isomeric forms, finally reaching an equilibrium between them. This is true for the simple sugar glucose, C6H12O6, which exists in solution in isomeric forms called alpha-glucose and beta-glucose. If a solution of glucose at a certain temperature is analyzed, and it is found that the concentration of alpha-glucose is twice the concentration of beta-glucose, what is the value of K for the inter-conversion reaction?arrow_forward12.101 An engineer working on a design to extract petroleum from a deep thermal reservoir wishes to capture toxic hydrogen sulfide gases present by reaction with aqueous iron(II) nitrate to form solid iron(II) sulfide. (a) Write the chemical equation for this process, assuming that it reaches equilibrium. (b) What is the equilibrium constant expression for this system? (c) How can the process be manipulated so that it does not reach equilibrium, allowing the continuous removal of hydrogen sulfide?arrow_forward12.100 A reaction important in smog formation is O3(g)+NO(g)O2(g)+NO2(g)K=6.01034 (a) If the initial concentrations are [O3]=1.0106M,[NO]=1.0105M,[NO2]=2.5104M, and [O2]=8.2103M , is the system at equilibrium? If not, in which direction does the reaction proceed? (b) If the temperature is increased, as on a very warm day, will the concentrations of the products increase or decrease? (HINT: You may have to calculate the enthalpy change for the reaction to find out if it is exothermic or endothermic.)arrow_forward
- In the figure, orange fish are placed in one aquarium and green fish in an adjoining aquarium. The two tanks are separated by a removable partition that is initially closed. (a) Describe what happens in the first few minutes after the partition is opened. (b) What would you expect to see several hours later? (c) How is this system analogous to dynamic chemical equilibrium?arrow_forwardWrite the equilibrium constant expression for each reaction in terms of activities, simplifying where appropriate. a C(s)+O2(g)CO2(g) b P4(s)+5O2(g)P4O10(s) c 2HNO2(g)+3Cl2(g)2NCl3(g)+H2(g)+2O2(g)arrow_forwardMustard gas, used in chemical warfare in World War I, has been found to be an effective agent in the chemotherapy of Hodgkin's disease. It can be produced according to the following reaction: SCl2(g)+2C2H4(g)S(CH2CH2Cl)2(g)An evacuated 5.0-1- flask at 20.0C is filled with 0.258 mol SCl2 and 0.592 mol C2H4. After equilibrium is established, 0.0349 mol mustard gas is present. (a) What is the partial pressure of each gas at equilibrium? (b) What is K at 20.0C?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning