
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
G.25.
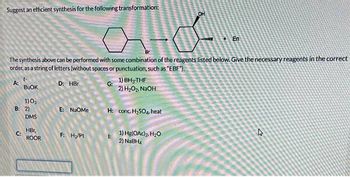
Transcribed Image Text:Suggest an efficient synthesis for the following transformation:
ato..
The synthesis above can be performed with some combination of the reagents listed below. Give the necessary reagents in the correct
order, as a string of letters (without spaces or punctuation, such as "EBF").
A:
t-
BUOK
C:
1) 03
B: 2)
DMS
HBr,
ROOR
D: HBr
E: NaOMe
F: H₂/Pt
G:
1) BH3-THF
2) H₂O₂, NaOH
H: conc. H₂SO4, heat
1:
OH
1) Hg(OAc)2, H₂O
2) NaBH4
+En
4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 11. A mixture was found to contain 4.36 g of NazCO3, 6.21g of S403 and 1.89 g of LI2Se: What is the percentage of Lithium selenium?arrow_forward(#26) Concentration of Solutions A concentrated solution of hydrochloric acid contains 120.0 g of water and 72.0 g of hydrochloric acid. (26a) What is the total moles of components in the final solution? Type-in your answer in Blank #1. Enter only the value in regular numerals with three significant figures. 26b) What is the mole fraction for hydrochloric acid? Type-in your answer in Blank #2. Enter only the value in regular numerals with no digit or space filler and with three significant figures. (26c) Given the density of the solution as 1.189 g/mL, what is the total volume of the solution? Type-in your answer in Blank #3. The unit is milliliters, but enter only the value in regular numerals with four significant figures (26d) What is the Molarity of the solution? Type-in your answer in Blank #4. The unit is mole/L but enter only the value in regular numerals with four significant figures. Dialarrow_forwardCalculation of AH; of Magnesium Oxide Mg + 2 HCI MgCl2 * Hz2 AH, = kJ/mole MgCl2 +H2O > Mgo + 2 HCI -AH2 = kJ/mole He + 를 O2 + H2O AH3 = - 286 kJ/mole net: Mg + 을 O2- MgO 사Hnet = kJ/mole The literature value for the AHf of magnesium oxide is -602 kJ/mole.arrow_forward
- 32. A lotion vehicle contains 15% v/v of glycerin (sp gr 1.25). How much glycerin should be used in preparing 5 gallons of the lotion? a. 2271 g glycerin b. 3339.7 mL glycerin c. 2671.8 g glycerin d. 3548.4 g glycerinarrow_forward5) If all the NaHCO3 in bag 2 reacted, calculate the number of moles of gas produced? tates) E Focusarrow_forward9. How many moles of 2 Na(s) * 24- 2. Provide an example calculation for row 8. This requires you to go from g CO2 to g NaHCO3 using stoichiometry. Use equation 2, a net ionic equation for the reaction of acid, H3O* (from acetic acid, CH3COOOH in vinegar) with the bicarbonate (from NaHCO3 in pill) to determine the molar ratio for your calculation. Note that Na+ (aq) doesn't show up in this equation because it is a spectator ion. Hag) Harrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY