
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
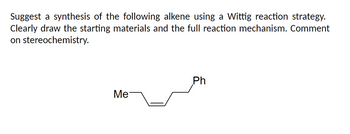
Transcribed Image Text:Suggest a synthesis of the following alkene using a Wittig reaction strategy.
Clearly draw the starting materials and the full reaction mechanism. Comment
on stereochemistry.
Me
Ph
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please do not draw the same proudct more than once in a reactionarrow_forwardAddition proceeds preferentially through the most stable carbocation intermediate. At high temperature, 10-20 °C or higher, products equilibrate; and the most stable product predominates (thermodynamic control). At low temperature, 0 °C or lower, there is kinetic control of reaction products. CI CI b Write a mechanism for the first step of this reaction using curved arrows to show electron reorganization. Consult the arrow-pushing instructions for the convention on regiospecific electrophilic attack on a double bond. Arrow-pushing Instructions n →XT H-CI:arrow_forwardDraw all of the substitution and elimination products formed from thegiven alkyl halide with each reagent: (a) CH3OH; (b) KOH. Indicate thestereochemistry around the stereogenic centers present in the products,as well as the mechanism by which each product is formed.arrow_forward
- Hydrogenation of alkene A with D2 in the presence of Pd-C affords asingle product B. Keeping this result in mind, what compound is formedwhen A is treated with each reagent: (a) mCPBA; (b) Br2, H2O followedby base? Explain these results.arrow_forwardGive clear detailed Solution with explanationarrow_forwardWhen treated with NaOH, the bromide below gives an alkene by the E2 mechanism, by eliminationof the circled atoms: (a) Draw the Newman projection from which elimination takes place. (b) Draw the mechanism. (c) Draw the product with the proper stereochemistry. (d) Assign the proper stereochemical descriptor to the product. (Z, E? Trans,CiS?) (e)What would happen to the stereochemistry of the product if the enantiomer of the starting material were used inthe reaction?arrow_forward
- addition of hbr to a double bond with an ether (-or) substituent occurs regiospecifically to give a product in which the Br OR are bonded to the same carbon. Draw the two possible carbocation intermediates in this electrophilic addition reaction,and explain using resonance why the observed product is formed.arrow_forwardDraw the structures of all possible products formed from the following reaction. Specifiy the type of substitution or elimination pathway. Demonstate the stereochemical outcomes of the reaction by drawing your products in wedge dash form when necessary.arrow_forwardF. a. Show a mechanism for the formation of SO3 from H₂SO4.arrow_forward
- Determine the mechanism of nucleophilic substitution for attached reaction anddraw the products, including stereochemistry.arrow_forwardFill in the missing reactants, reagents and products in the following reactions. Indicate stereochemistry if necessary. Unless otherwise specified, assume the reagents are in excess. PLease explaion with MUCH detail.arrow_forwardIdentify compounds A – E of the reaction sequence shown in Scheme III, making sure to include stereochemistry as appropriate.a) Identify compound A in Scheme III. b) Identify compound B in Scheme III. c) Identify compound C in Scheme III. d) Identify compound D in Scheme III. e) Identify compound E in Scheme III.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning