
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Need help on drawing the first step of the mechanism. Including the formal charges and lone pairs
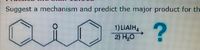
Transcribed Image Text:Suggest a mechanism and predict the major product for th
1) LIAIH,
2) H0

Transcribed Image Text:Provide the missing curved arrow(s) to show the first step in the mechanism. Modify the given structure to draw the intermediate formed in the
first step. Include formal charges and lone pairs in your answer.
楽東 東東產 東 新
海:
連
臺 美
H.
II
S.
H-7-A--H
Close
CI
Br
\ Ź + 1 +1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Pick the one reaction whose products don't match the given arrows. Assume lone pairs and formal charges are correct. A B C D H 07:0 H : OCH 3 :F: H-CI: 61: HO. :OCH 3 :ךד: + F: 어 HOTarrow_forwardDraw the resonance structures for the following: ) Please use curved arrows to show how you push the electron(s), and add formal charges.arrow_forwardElectron Groups shape (EGs) 2. Total smallest 90° 120° 180° number angle of bonded atoms shape (B.P) + lone pairs (L.P) on smallest centre 90° 109° 120° angle atom Discuss with your neighbours and provide answers to the following questions. Circle the words that make each statement correct. 1) Each row represents different possible arrangements of that number of electron groups (EG). In each structure, the EGs get [ closer together / further apart ] and the angles between them get [smaller /larger ]. 2) The observed structures adopted by real molecules are those in the thick borders. These structure have the [ smallest / largest ] angles and the EGs are the [ closest together | furthest apart ]. 3) The preferred structure is adopted because this [ minimizes / maximizes ] the [ repulsion / attraction ] felt between all EGs. (Clue: what does VSEPR stand for?) 3.arrow_forward
- 2. On the first drawing on the left for each structure below, draw the electron pushing arrows needed to produce the resonance structure on the right. The structures are bond-line; you need to draw the implied lone pair electrons that participate in resonance. (b) (a) Ⓒarrow_forward7. Now let's combine our knowledge of bond-line formulas and formal charge. How many H-atoms are on the carbon atoms containing the 1+ formal charge? H-atoms H-atoms H-atomsarrow_forward3) Draw all possible resonance structures for the following. For each molecule If any appear significantly less stable, circle it, iv) 0 EN:arrow_forward
- Use curved arrows to show the movement of electron pairs in attached reaction.arrow_forward1. What type of reaction that all organic compounds undergo? 2. What is the total bond order of sulfur in CH3SCH3? 3. Explain the meaning in organic formulas of a pair of parentheses with no subscript behind it, such as in CH3CH2CH(CH3)C3H7arrow_forward12. Provide one other contributing resonance form for structures A, B, and C. Show all electron movement using curved arrows. Between the two structures, circle the higher contributing resonance form. A В C HO.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY