
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Single displacement redox
Transcribed Image Text:Herrera - Leam X
www-awa.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-lvgXwPgmUhvITCeeBZbufu BYTHz7m7D3ZcSLUw
O ELECTROCHEMISTRY
Writing the half-reactions of a single-displacement reaction
Grades for Andrew Herrera: CHEN X +
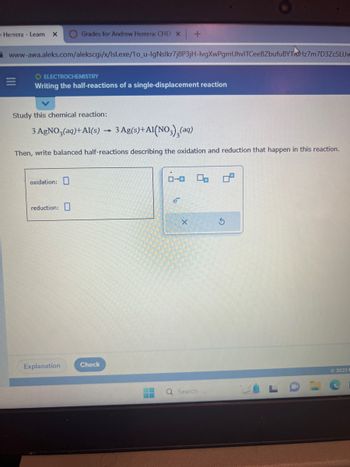
Study this chemical reaction:
3 AgNO3(aq) +Al(s) 3 Ag(s) + A1(NO3)₂(aq)
Then, write balanced half-reactions describing the oxidation and reduction that happen in this reaction.
oxidation:
reduction:
Explanation
Check
1
e
X
Q Search
2023 F
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- the half reaction method can be used to simplify equations. true or falsearrow_forwardTreatment of gold metal with BrFg and KF produces Bro and KAuFs, a salt of gold. Based on this information Mis oxidized and is reducedarrow_forwardII. GENERAL INSTRUCTIONS Read and analyze carefully each problem. Show your complete solution. 1. Balance each redox reaction in basic medium. Don't forget to identify the oxidation half- reaction and reduction half-reaction. b. Te + NO Teo]¯ + N₂0₂ c. 105 + Re-Re0 + 10arrow_forward
- Consider the unbalanced redox reaction shown below: Mn+2 + BiO3-1 + H+1 → MnO4-1 + Bi+3 + H2O What is the oxidizing agent?arrow_forwardThe following fictitious unbalanced REDOX reaction is made of some teachers initials and some Oxygen. Balance the following REDOX reaction on a piece of paper and show ALL EIGHT steps. Ch2+ + ShO4– Sh2+ + Ch3+ (acidic solutionarrow_forwardThe electrode at which _________ occurs is called the cathode and the electrode where the ____________ occurs is called the anode Oxidation, neutralization Reduction, neutralization Reduction, oxidation Oxidation ,reductionarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY