
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
study the graph
What is true according to the information in the graph?
A) The enthalpy of the reaction is positive.
B) The reaction is an exothermic reaction.
C) The reaction releases energy to its surroundings.
D) The enthalpy of the reaction is negative.
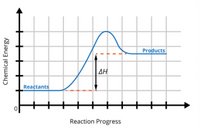
Transcribed Image Text:Products
ΔΗ
Reactants
Reaction Progress
Chemical Energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The Haber process is exothermic. (the formation of NH3 from its elements) a) Write out the balanced chemical equation (with subscripts) and include energy.arrow_forwardHello, can you please solve this question and please show all your work. Thank you. It's one questions with two parts. Please solve botharrow_forwardPlease help me answer this questionarrow_forward
- %24 An 33.777g piece of metal is heated in a hot water bath at 98.5°C and then transferred to a coffee-cup calorimeter containing 50.0mL of water. The initial water temperature was 22.9°C and the final water temperature was 27.3°C. Determine the specific heat of the metal (Cmetal). Ccal = 21.0J/°C & Cwater = 4.184 J/g*°C. Report your answer to 3 sf and in J/g*°C. Your Answer: Answer units MacBook Air DII DD 000 吕0 F9 F10 F7 F8 F6 F5 F3 F4 * #3 7 8 9 4arrow_forwardWhen 1.42 g of iron reacts with 1.80 g of chlorine, 3.22 g of iron(II) chloride (FeCl2, molar mass = 126.75 g/mol) and 6.8 kJ of heat is produced. What is the enthalpy change for the reaction when 1 mole of iron(II) chloride is produced? round to sig figsarrow_forwardIf 87 J of heat are added to a balloon, causing it to expand from 1.0 L to 1.4 L under 0.97 atm of pressure, what is the change in energy of the balloon (in joules)?arrow_forward
- When two solutions are combined in a test tube, they quickly react and the test tube containing the reaction becomes instantly hot to the touch. Is this reaction exothermic or endothermic? Briefly explain how you know.arrow_forwardFor an exothermic reaction, how is the measured temperature change affected if some of the heat produced transfers to the calorimeter? How will the determination of the specific heat of the metal be affected if the thermometer used to record the temperature of the water bath reads too low?arrow_forwardAn unknown 10 gram sample in a lab required 44.9 Joules of energy to change the temperature 10 degrees Celsius. What was the specific heat of this element? Using the specific heat, find the likely element using your Reference Table.arrow_forward
- During an endothermic chemical reaction, a system becomes [Select] [Select] in potential energy. and the chemical substances undergo anarrow_forwardIn an experiment, 26.5 g of metal was heated to 98.0°C and then quickly transferred to 150.0 g of water in a calorimeter. The initial temperature of the water was 22.5°C, and the final temperature after the addition of the metal was 32.5°C. Assume the calorimeter behaves ideally and does not absorb or release heat.arrow_forwardRachel West Section 017 A thermometer placed in a solution undergoing a chemical reaction indicates an increase in temperature as ie reaction proceeds. Is this reaction endorhermic or exothermic? Describe if heat energy is lost or gained Trom the reaction (the system) to the surroundings. What is the sign of the enthalpy change (AH) of this reaction? A student performs a reaction and determines the enthalpy change (AH) to be 31.4 kJ. Will the cemperature of the surrounding solution increase or decrease as a result of this chemical process?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY