Question
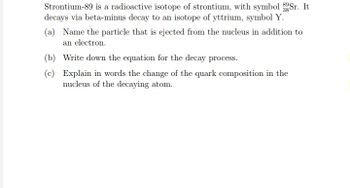
Transcribed Image Text:Strontium-89 is a radioactive isotope of strontium, with symbol 3gSr. It
decays via beta-minus decay to an isotope of yttrium, symbol Y.
(a) Name the particle that is ejected from the nucleus in addition to
an electron.
(b) Write down the equation for the decay process.
(c) Explain in words the change of the quark composition in the
nucleus of the decaying atom.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Consider the following a-decay of the Uranium nucleus 236 U → 332Th + a. 90 (a) Show how the mass number (Aa) and atomic number (Za) of the alpha particle are obtained from this equation. (b) Calculate the Q-value (Qa) of the reaction. (c) Calculate the speed va = √2MQ of the alpha particle after it has been ejected from the parent nucleus, in terms of the speed of light c. M = -, mp and ma are the atomic masses (ma+mp) x ma mp of the daugher nucleus and the alpha particle, respectively. (d) Calculate the classical turning radius Re= 2Zpe²/Qa, where Zp is the atomic number of the daughter nucleus, and e² = 1.44 MeV. fm. (e) Calculate the decay probability Pa that the alpha particle will tunnel through the barrier. HINT: In calculating the probability, use the fact that ħc=197.327 MeV fm. The formula to use is given on page 5.arrow_forwardWhat is the nuclear equation for 2Pu (alpha decay) wwwwwww 244PU 242 90 1) 244 Pu → 24QU + ¿He 92 248 c. 2Pu -> 94 244Pu → 246TH + ZHe 94 90arrow_forward7 mi.. of. tr. J. 0.. J.. D. e.. 8 Al 32. A baryon is a hadron composed of THREE quarks. A neutron is a baryon with the following quark designation: cbb Oudd Duud Ouss 33. Which of the following decay equations describes beta positive decay? Ouududd +e++v Oudd uud+e++v Oudduud +e+v Ouududd +e+v Written Response 34. hp BB afe FAarrow_forward
- Please answer this within 30 mins ! I will upvote !arrow_forwardA certain particle has a half-life of 179. s. What is the decay constant, in s-1?arrow_forwardA new element has been created artificially. It is named Elementy McFacium. The most common isotope of McFacium has 153 protons and 277 neutrons. The mass of this isotope of the new element is 402,188.91 MeV/c^2. What is the total mass deficit for the nucleus? Hint: The new element is not made as an ion! Use the mass of hydrogen atoms instead of protons. (Answer in atomic mass units u)arrow_forward
- A state-of-the-art proton decay experiment is expected to detect 47% of the proton decays in a body of water. Assuming protons have a lifetime of 1031 years, how many m3 of water would you need in order to see 6 decays per month? (Assume a "month" is one-twelfth of a year.)arrow_forwardOutside the nucleus, the neutron itself is radioactive and decays into a proton, an electron, and an antineutrino. The half-life of a neutron (mass = 1.675 × 10-27 kg) outside the nucleus is 10.4 min. On average, over what distance x would a beam of 7.04-eV neutrons travel before the number of neutrons decreased to 75.0% of its initial value? Ignore relativistic effects. X = iarrow_forwardThe half-life of 198Au is 2.70 days. What is the decay constant of 198Au?arrow_forward
arrow_back_ios
arrow_forward_ios