
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
What is the mass flow rate of cadmium (ṁCd,3) in kg/h in the liquid stream to 1 decimal place?
What is the mass fraction of zinc (xZn,2) in the gas stream to 1 decimal place?
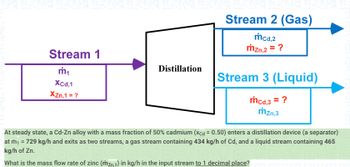
Transcribed Image Text:Stream 2 (Gas)
mcd.2
mzn.2 = ?
Stream 1
Distillation
m₁
Stream 3 (Liquid)
Xcd,1
XZn,1 = ?
mcd,3 = ?
mzn.3
At steady state, a Cd-Zn alloy with a mass fraction of 50% cadmium (xcd = 0.50) enters a distillation device (a separator)
at m₁ = 729 kg/h and exits as two streams, a gas stream containing 434 kg/h of Cd, and a liquid stream containing 465
kg/h of Zn.
What is the mass flow rate of zinc (mzn,1) in kg/h in the input stream to 1 decimal place?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Need asap show complete solutionarrow_forwardFind the mass flow rate of streams 5 (ṁ5) in kg/h to 1 decimal place. Find the mass flow rate of streams 4 (ṁ4) in kg/h to 1 decimal place. Find the mass flow rate of streams 6 (ṁ6) in kg/h to 1 decimal place. What is the mass flow rate of stream 2 (ṁ2) in kg/h to 1 decimal place? What is the mass fraction of KCl in stream 2 (xKCl,2) to 2 decimal places? What is the mass fraction of water in stream 2 (xH₂O,2) to 2 decimal places?arrow_forwardA lake with 100 x 107 m² of surface area is fed by a stream having 45 m³/s of flow with 2.3 mg/L phosphorus. Phosphorus is also entering the lake through an industrial wastewater treatment plant discharges at 1.4 m³/s containing 235 mg/L phosphorus. If the lake is well mixed and the phosphorus settling rate is 13 m/yr, estimate the steady-state phosphorus concentration in the lake. What level of phosphorus removal at the treatment plant would be required to keep the average phosphorus concentration in the lake below 0.30 mg/L?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The