
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
![In industry, it is logical that the most expensive and the
rarest chemical must be chosen as the
[Select]
+](https://content.bartleby.com/qna-images/question/be53d701-6691-4314-9bda-29e504eeb7d9/88c8f349-7de2-4d61-81ae-3a4cef36c55f/0kyjdp7_thumbnail.jpeg)
Transcribed Image Text:In industry, it is logical that the most expensive and the
rarest chemical must be chosen as the
[Select]
+
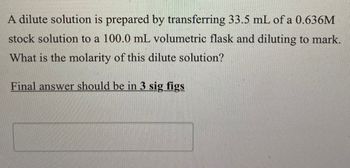
Transcribed Image Text:A dilute solution is prepared by transferring 33.5 mL of a 0.636M
stock solution to a 100.0 mL volumetric flask and diluting to mark.
What is the molarity of this dilute solution?
Final answer should be in 3 sig figs
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 100.0-mL solution containing 0.500 moles of NaCl is mixed with 200.0 mL of water. What is the molarity of the resulting solution?arrow_forwardYou may want to reference (Pages 306 - 308) Section 9.5 while completing this problem. Part A Determine the volume, in milliliters, required to prepare each of the following diluted solutions. 23.0 mL of a 0.250 M KNO3 solution from a 6.50 M KNO3 solution Express your answer with the appropriate units. HÁ Value Units Submit Request Answer Part B 28.0 mL of a 2.50 M H2SO4 solution from a(n) 12.4 M H2SO4 solution Express your answer with the appropriate units. HA ? Value Unitsarrow_forwardIf 151 mL of a solution of HCl are diluted to 853 mL of 0.100 M HCl, what is the molarity of the original HCl solution? Enter only the numeric value for your answer (no units).arrow_forward
- 101) X -p.101edu.co - Hiring Ce... F4 144 $ 4 Aktiv Chemistry F5 PU 25 F6 A k How many moles of sodium chloride are there in 0.55 L of a 1.60 M aqueous solution of sodium chloride? A 6 X A) 2.90 Login - Labster B) 0.344 C) 0.880 F7 D) 4.20 F8 7 X Question 9 of 9 F9 88 11. Erikson's Psychosocial Theory o X DELL F10 9 F11 8 O F12 + PrtScr T DE Insert Dele Backarrow_forwardMasses : Ca = 40.08 g S= 32.07 g O= 16.00 g A 35.0 mL sample of CaSO4 was evaporated to dryness, leaving 0.967 g of residue. What was the molarity of the original solution? step by step pleasearrow_forwardWhat volume, in milliliters, of 14.6 M HCl is required to make 68.3 mL of 3.16 M HCl? Type only the numerical answer - no units.arrow_forward
- With the correct amount of sig figs thank you!arrow_forwardIf you dilute 27.0 mL of the stock solution from part a) (the answer is 6.25) to a final volume of 1.00 L, what will be the concentration of the diluted solution?arrow_forward15.0 mL of 17.2 M NaNO3 is diluted to 100.0 mL. What is the molarity of the new solution? __________ M (Report your answer with the correct sig figs in units of moles/ L, Marrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY